Catalyst for producing hydrogen and method for producing hydrogen
a catalyst and hydrogen technology, applied in the direction of metal/metal-oxide/metal-hydroxide catalysts, electrochemical generators, physical/chemical process catalysts, etc., can solve the problems of failing to produce a mayenite-type structure and the method of supplying hydrogen to fuel cells, and achieve low cost, high clarke number, and high weight hourly space velocity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of C12A7 Base Material Containing Oxygen Ions
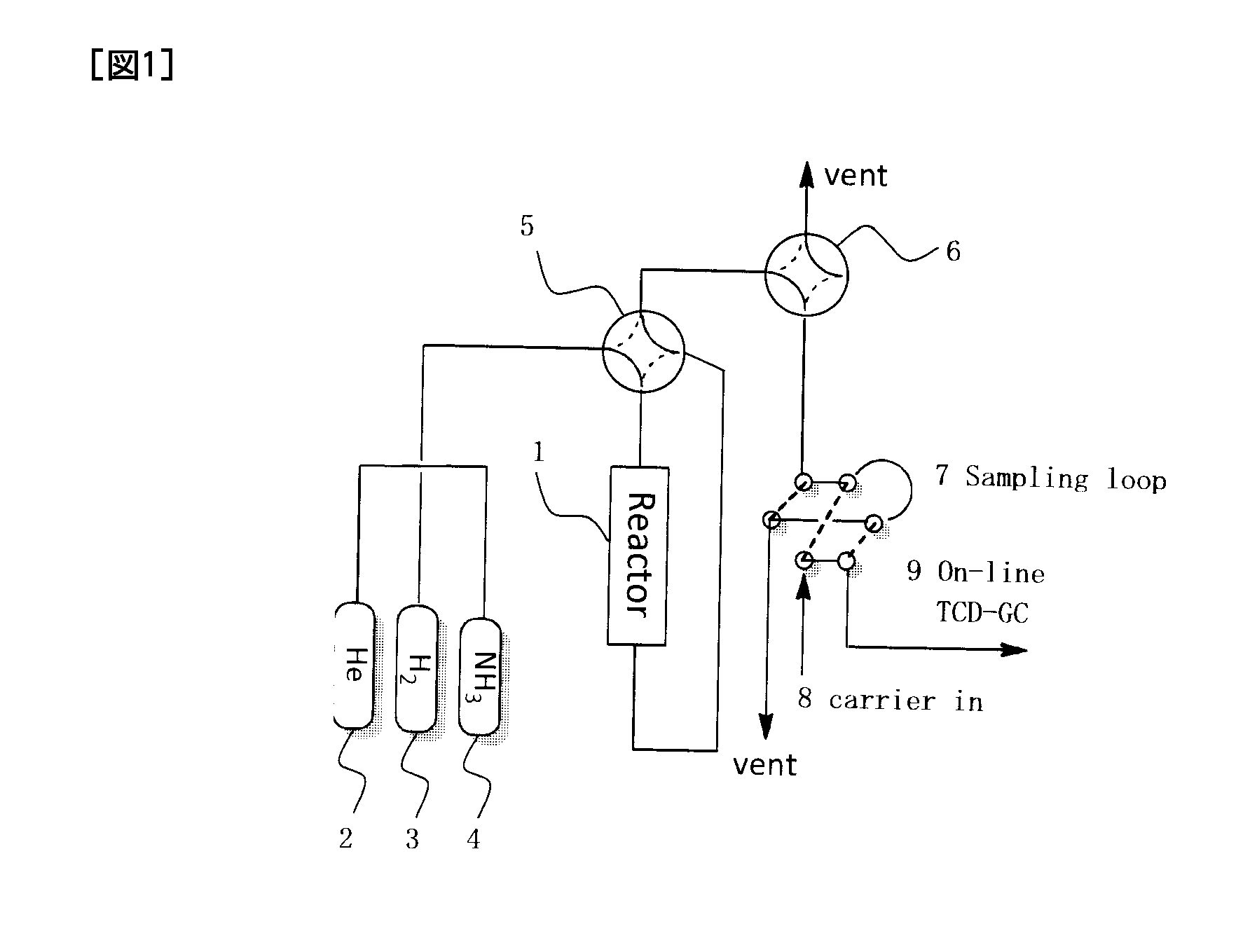
[0082]Powders of Ca(OH)2 (Shuzui Hikotaro Shoten, 23.1 g) and Al(OH)3 (Kojundo Chemical Laboratory Co., Ltd., 28.4 g) were mixed so that a Ca / Al molar ratio was 12:14, and 449 ml of water was added to the resultant mixture, followed by hydrothermal treatment in an autoclave at 150° C. over 5 hours. The resultant powder was filtered, washed with 500 ml of water, dried at 150° C., fired in an oxygen stream at 800° C. for 2 hours, and then ground to prepare a C12A7 (referred to as “C12A7:O” hereinafter) powder having a specific surface area of 40 m2g−1 and a particle diameter of 0.1 mm to 0.5 mm and containing oxygen ions but not containing conduction electrons and hydrogen anions.
[0083]The C12A7:O powder prepared by the method described above was inserted into a silica glass tube and pre-treated by vacuum-heating at 800° C. for 15 hours in a vacuum of 1×10−4 Pa. Then, 2.5 g of the resultant powder was inserted, together with 0.1...
example 2
[0087]A 5 wt % Ru / C12A7:e powder was prepared by the same method as in Example 1 except that the amount of Ru supported was 5 wt %, and ammonia decomposition reaction was carried out. The results are shown in Table 1. The NH3 conversion rate at 440° C. was 67.2%, and the NH3 decomposition rate at 440° C. was 6.9 (kgNH3kgcat−1h−1).
example 3
[0088]A 2 wt % Ru / C12A7:H powder was prepared by the same method as in Example 1 except that C12A7:H was used as a support, and ammonia decomposition reaction was carried out. The results are shown in Table 1. The NH3 conversion rate at 440° C. was 76.5%, and the NH3 decomposition rate at 440° C. was 7.9 (kgNH3kgcat−1h−1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| BET specific surface area | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com