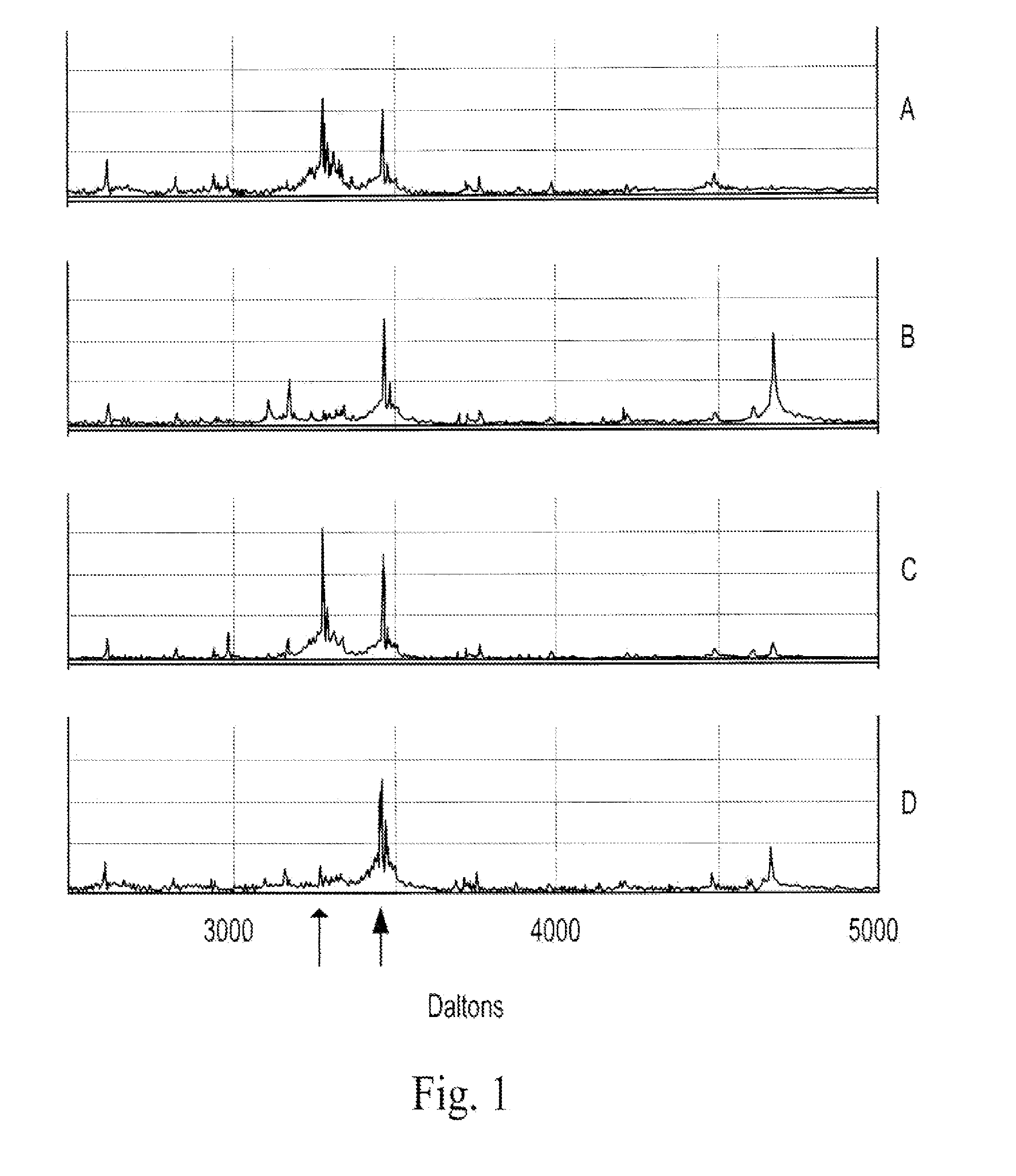
Compositions and methods for treating cardiovascular disease and myocardial infarction with dipeptidyl peptidase inhibitors or b type natriuretic peptide analogues resistant to prolyl-specific dipeptidyl degradation
a technology of dipeptides and analogues, which is applied in the field of medical diagnostics and therapeutics, to achieve the effects of improving diagnostic and prognostic information, good stability, and facilitating patient treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0177]Blood is preferably collected by venous puncture using a 20 gauge multi-sample needle and evacuated tubes, although fingertip puncture, plantar surface puncture, earlobe puncture, etc., may suffice for small volumes. For whole blood collection, blood specimens are collected by trained study personnel in EDTA-containing blood collection tubes. For serum collection, blood specimens are collected by trained study personnel in thrombin-containing blood collection tubes. Blood is allowed to clot for 5-10 minutes, and serum is separated from insoluble material by centrifugation. For plasma collection, blood specimens are collected by trained study personnel in citrate-containing blood collection tubes and centrifuged for ≧12 minutes. Samples may be kept at 4° C. until use, or frozen at −20° C. or colder for longer term storage. Whole blood is preferably not frozen.
example 2
Recombinant Antibody Preparation
[0178]Immunization of Mice with Antigens and Purification of RNA from Mouse Spleens
[0179]Mice are immunized by the following method based on experience of the timing of spleen harvest for optimal recovery of mRNA coding for antibody. Two species of mice are used: Balb / c (Charles River Laboratories, Wilmington, Mass.) and A / J (Jackson Laboratories, Bar Harbor, Me.). Each of ten mice are immunized intraperitoneally with antigen using 50 μg protein in Freund's complete adjuvant on day 0, and day 28. Tests bleeds of mice are obtained through puncture of the retro-orbital sinus. If, by testing the titers, they are deemed high by ELISA using biotinylated antigen immobilized via streptavidin, the mice are boosted with 50 μg of protein on day 70, 71 and 72, with subsequent sacrifice and splenectomy on day 77. If titers of antibody are not deemed satisfactory, mice are boosted with 50 μg antigen on day 56 and a test bleed taken on day 63. If satisfactory titer...
example 3
Biochemical Analyses
[0222]BNP is measured using standard immunoassay techniques. These techniques involve the use of antibodies to specifically bind the protein targets. An antibody directed against BNP is biotinylated using N-hydroxysuccinimide biotin (NHS-biotin) at a ratio of about 5 NHS-biotin moieties per antibody. The biotinylated antibody is then added to wells of a standard avidin 384 well microtiter plate, and biotinylated antibody not bound to the plate is removed. This formed an anti-BNP solid phase in the microtiter plate. Another anti-BNP antibody is conjugated to alkaline phosphatase using standard techniques, using SMCC and SPDP (Pierce, Rockford, Ill.). The immunoassays are performed on a TECAN Genesis RSP 200 / 8 Workstation. Test samples (10 μL) are pipetted into the microtiter plate wells, and incubated for 60 min. The sample is then removed and the wells washed with a wash buffer, consisting of 20 mM borate (pH 7.42) containing 150 mM NaCl, 0.1% sodium azide, and 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com