Optimization of antibodies that bind lymphocyte activation gene-3 (lag-3), and uses thereof
a technology of lymphocyte activation gene and antibody, applied in the field of optimizing antibodies, can solve the problems of reducing the effect of antibody amide content, affecting the ability of antibodies to bind, and causing life-threatening side effects, etc., and achieves improved thermal and chemical stability, improved physical stability, and improved stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of Variants of LAG3.1 (Antibody 25F7)
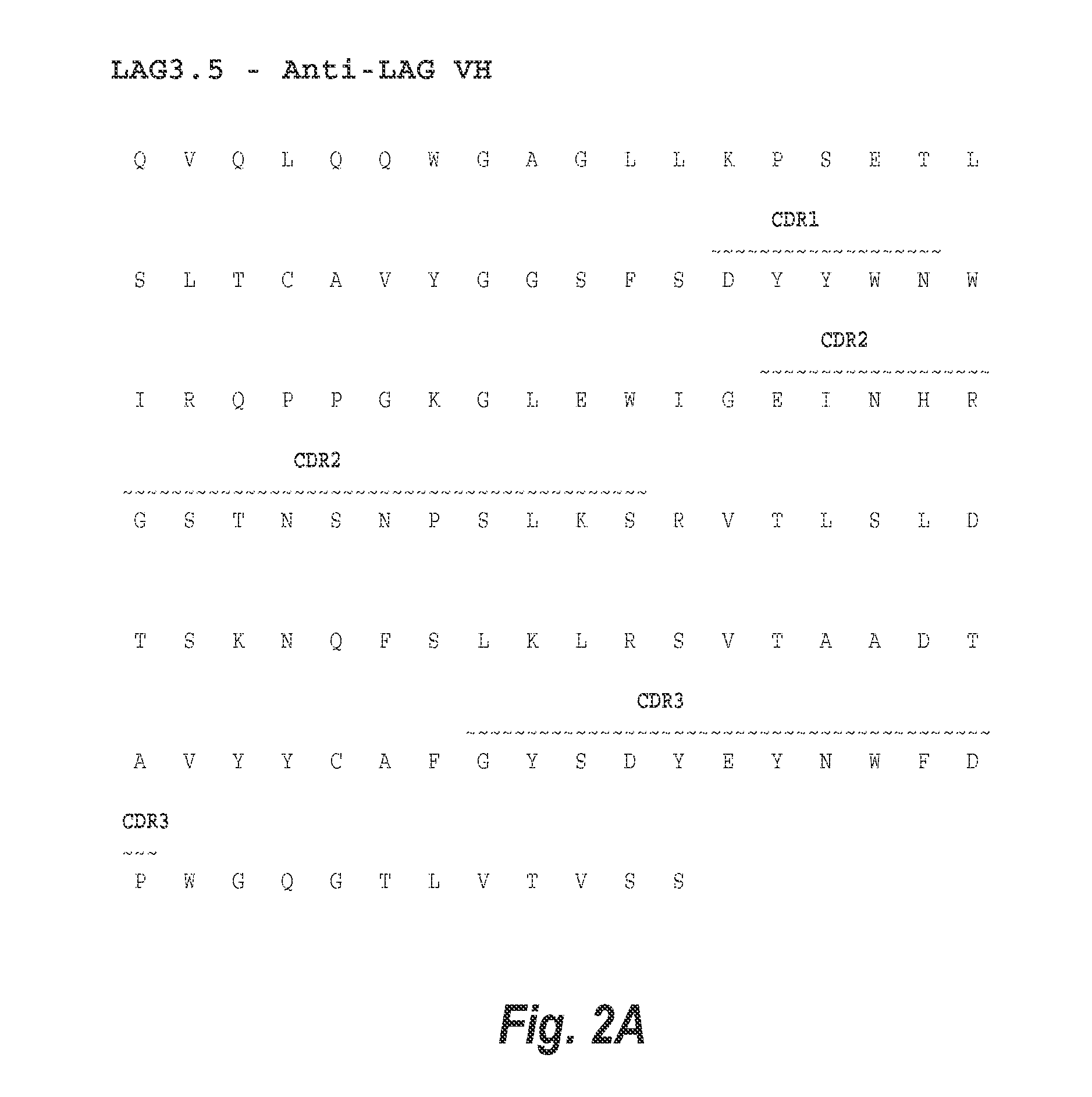
[0219]Antibody variants of the previously described anti-LAG-3 antibody, 25F7, referred to herein as LAG3.1, were created by first analyzing the amino acid sequence of the antibody for potential sites of degradation. Expression of site-directed mutagenesis of LAG3.1 VH region was performed using QuikChange II XL® Site-Directed Mutagenesis Kit (Agilent Technologies). The altered VH regions were then subcloned into UCOE® (EMD Millipore) vectors that contain the human IgG4-S228P constant region. The various heavy chain vectors were each co-transfected with a vector expressing the LAG3.1 kappa chain into CHO-S cells, and stable pools were selected for expression.
[0220]Five potential deamidation motifs were identified within the variable region heavy chain CDR2. These sites were located at positions 52, 54, 56, 58, and 60 of the heavy chain variable region of LAG3.1 (SEQ ID NO: 2) (see FIG. 1A). In particular, deamidation of the “NG” sequence w...
example 2
Characterization of LAG-3 Variants
[0222]1. Activated human CD4+ T Cell Binding To test the ability of the antibody variants to bind to native human LAG-3 on the surface of activated human T cells, normal healthy donor peripheral blood mononuclear cells were stimulated in 15 cm tissue culture plates at a density of 2×10e6 cells / mL, with a combination of anti-CD3 (eBioscience, Cat #16-0037-85) and anti-CD28 (BD Bioscience, Cat #555725) antibodies present in solution at 5 μg / mL and 3 μg / mL, respectively. Following three days of stimulation cells were harvested, washed 1× with 1×PFAE buffer (1×PBS+2% FBS, 0.02% sodium azide, 2 mM Na EDTA), and resuspended in 1×PFAE buffer for staining.
[0223]For the binding reaction, the LAG3.1 variants were serially diluted with cold 1×PFAE buffer, then 50 μl of diluted antibody solution was mixed with 50 μl of Fitc-labeled anti-human CD4 (BD Bioscience, Cat #555346) diluted 1:16 in 1×PFAE buffer. For the binding reaction, 100 μl of this diluted antibod...
example 3
Variant Selection
[0232]Based on the studies described above, antibody variant LAG3.5 was selected for further analysis, in view of its significantly improved physical and chemical stability compared to its unmodified form (LAG3.1), particularly its high capacity for conformational refolding (thermal reversibility). This analysis included a two-step approach of (a) accelerated stress, (b) followed by 12-week real-time stability evaluation. Specifically, LAG3.5 was incubated at 1.0 mg / ml in pH 8.0, 50 mM Ammonium Bicarbonate, for 5 days at 40C.° The degree of modifications after 5 days was analyzed, as well as the effects on activity and stability. The LAG3.5 variant was then subjected to real-time stability in PBS for a duration of 12 weeks and subsequently analyzed. The results of these studies are described below.
[0233]1. Antigen Binding
[0234]As shown in FIG. 7 (and Table 5), no change in antigen binding was observed after 5 days. As also shown in FIGS. 10 A and B, LAG3.5 exhibited...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com