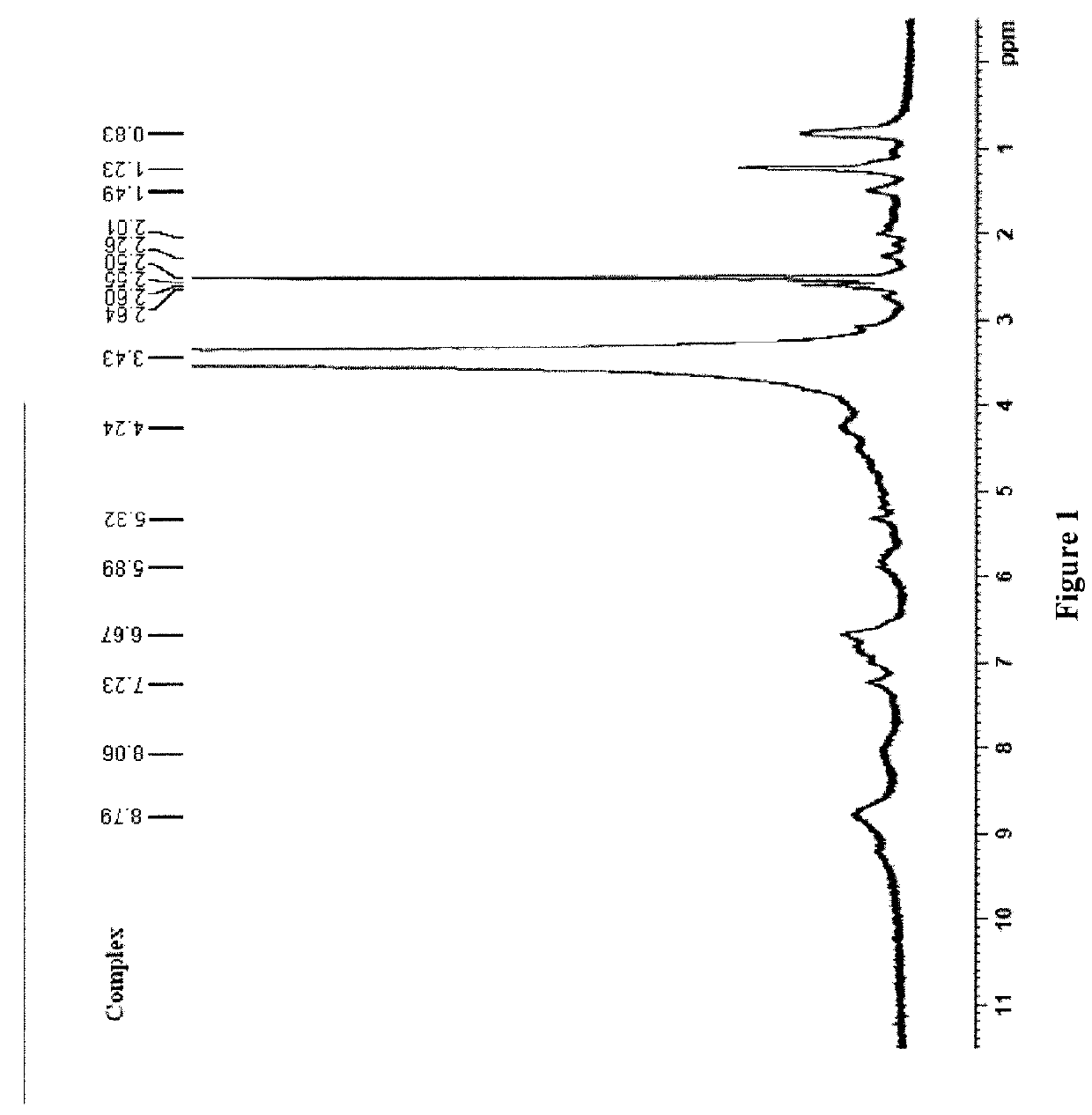
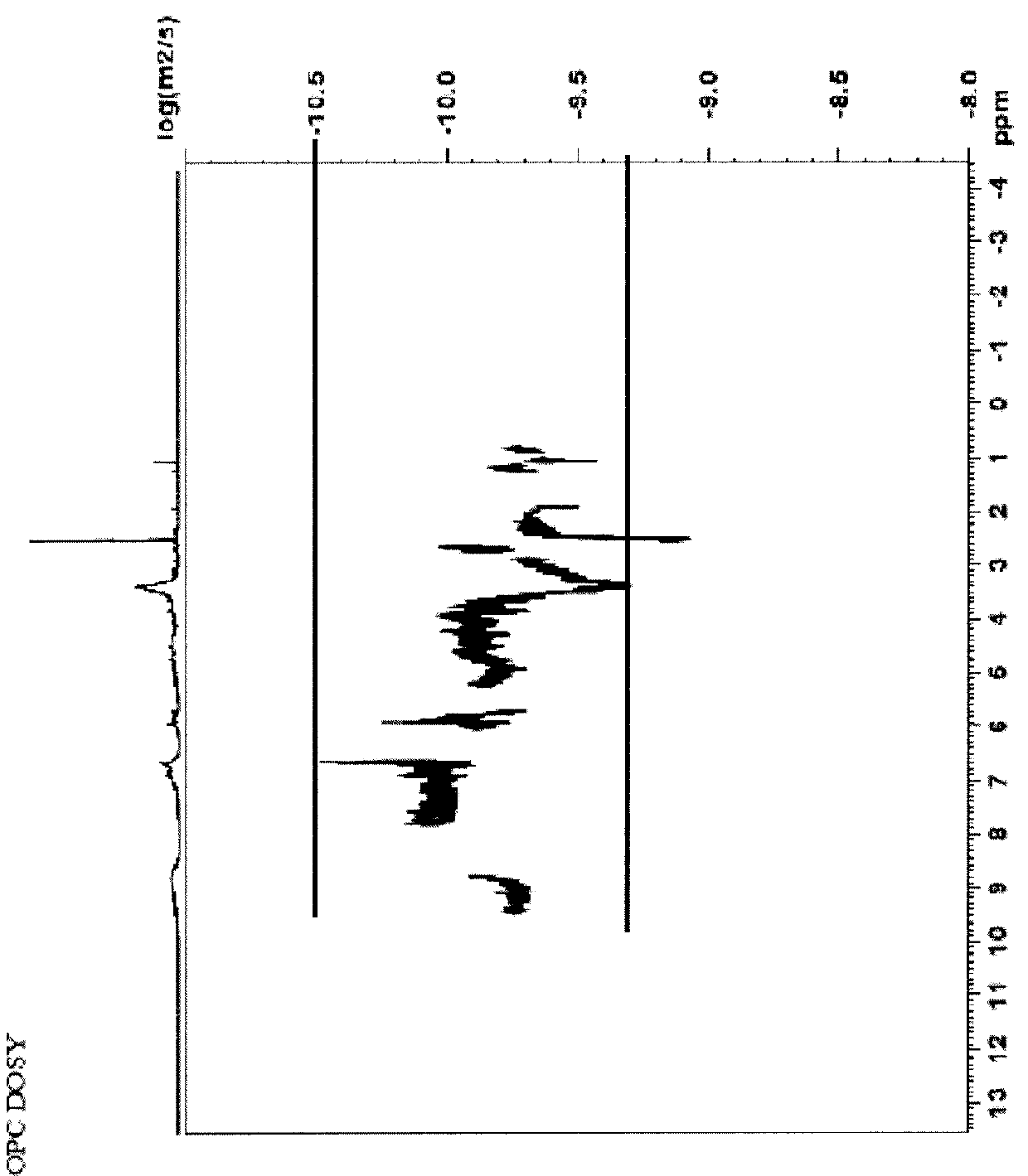
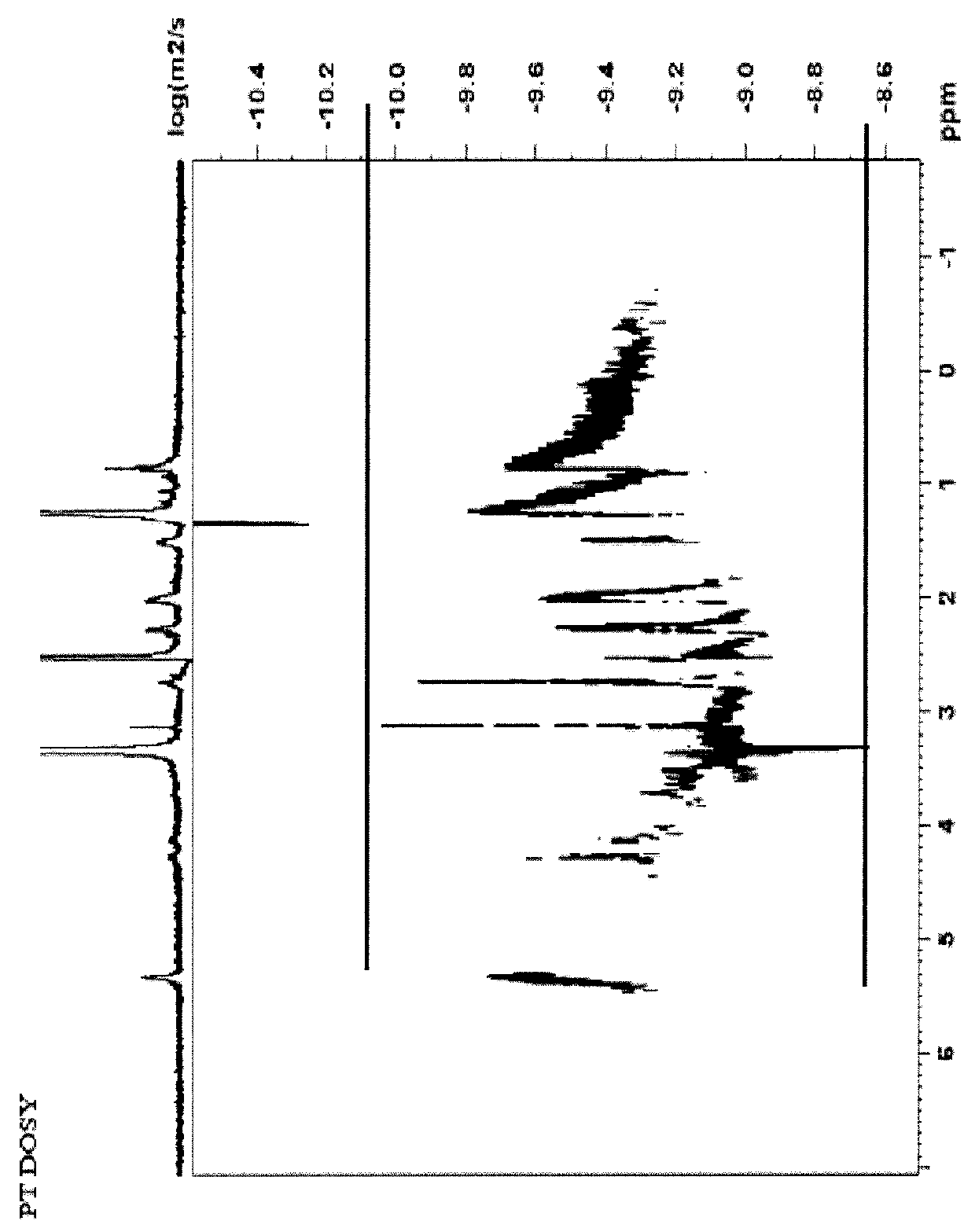
Compositions comprising complexes of proanthocyanidins with vegetable proteins
a technology of proanthocyanidins and complexes, which is applied in the field of complexes comprising proanthocyanidins and vegetable proteins, can solve the problems of complexes with animal proteins that can also give rise to ethical or religious problems in some patient populations, and are incapacity and dangerous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Complex
[0029]A) 10 g of pea protein (Dal Cin Gildo S.p.A.) is suspended in 200 g purified water. The pH of the suspension is adjusted from pH 6.3 to pH 4 with anhydrous citric acid, 20% sol. w / v in water. The suspension is left under stirring for 3 hours, and the temperature is increased to 30° C.
[0030]B) 10 g of proanthocyanidins (Grape Seed Dry Extract®, Indena Spa) is dissolved in 100 ml of purified water; the solution is prepared shortly before use, adjusting the pH from 6.7 to 4 with anhydrous citric acid, 20% sol. w / v in water, and heated to 30° C.; the dark red solution is added in portions to suspension A), still under stirring.
[0031]The complex in homogenous suspension that immediately forms is dark red, while the aqueous solution is reddish.
[0032]The suspension is kept under stirring for 3 hours, leaving the temperature to fall to 20° C.
[0033]Stirring is stopped and the suspension is left to stand for a further 8 hours, during which time it rapidly decants. ...
example 2
Biological Tests in Vitro
[0047]The efficiency of the barrier and the chemical stability of the complex of the invention were evaluated by comparison with commercial gelatin tannate (Tasectan®) in the intestinal epithelium using the well-established predictive model of Caco-2 cells, a cell line of the intestinal epithelium deriving from a colorectal adenocarcinoma (ATCC HTB 37) (Cell. Biol. Toxicol. 2005, 21(1) 1-26).
[0048]The paracellular flow and the barrier permeability of the compounds tested were determined.
[0049]Paracellular flow was determined by measuring the transepithelial electrical resistance (TEER), which provides a direct measurement of the barrier function and is a further parameter of the integrity of the barrier at the tight junctions.
[0050]The permeability of the barrier was measured by determining the passage of the Lucifer Yellow dye (Le Ferrec et al., Altern Lab Anim. 2001 November-December; 29(6):649-68; Hidalgo et al., Gastroenterology. 1989 March; 96(3):736-49...
example 3
Biological Tests in Vivo
[0053]The activity of the complex of proanthocyanidin obtained from Vitis vinifera and pea protein on the altered intestinal permeability and intestinal inflammation induced by lipopolysaccharide (LPS) was evaluated.
[0054]Groups of 8 male Wistar rats (200-225 g) have been used. After on overnight fast, the animals were injected intraperitoneally (IP) with 250 μL of sterile saline (NaCl 0.9%) containing or not (control) 1 mg / kg of lipopolysaccharide (LPS) from E. Coli. This dose has been previously shown to alter intestinal permeability and to release pro-inflammatory cytokines in the mucosa (TNFα, IL-1β, IFNγ (Moriez R,. Am J Pathol. 2005; 167(4):1071-9). Six hours later, the animals have been sacrificed and strips of jejunum were used for evaluation of TEER and FITC-dextran paracellular permeability. Other segments were also collected for other parameters of mucosal inflammation (myeloperoxidase-MPO).
[0055]Six hours after LPS administration, the rats were sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com