Stents modified with material comprising amnion tissue and corresponding processes
a technology of amnion tissue and stents, which is applied in the field of amnion tissue to modify stents, can solve the problems of delayed re-endothelization, drug eluting stents, and known risks of implantation of stents, and achieves the effects of reducing the risk of restenosis, and reducing the risk of stent ejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
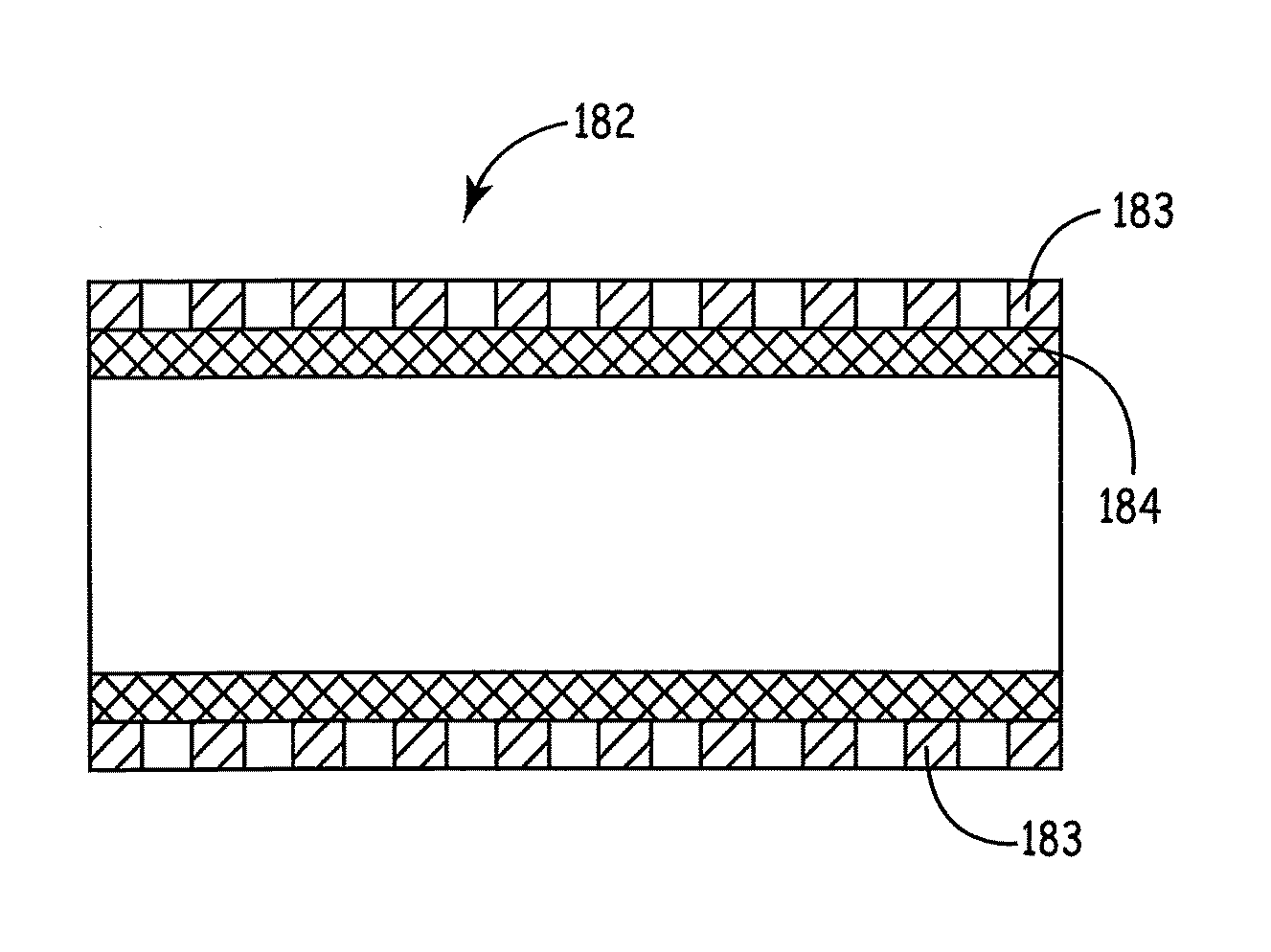
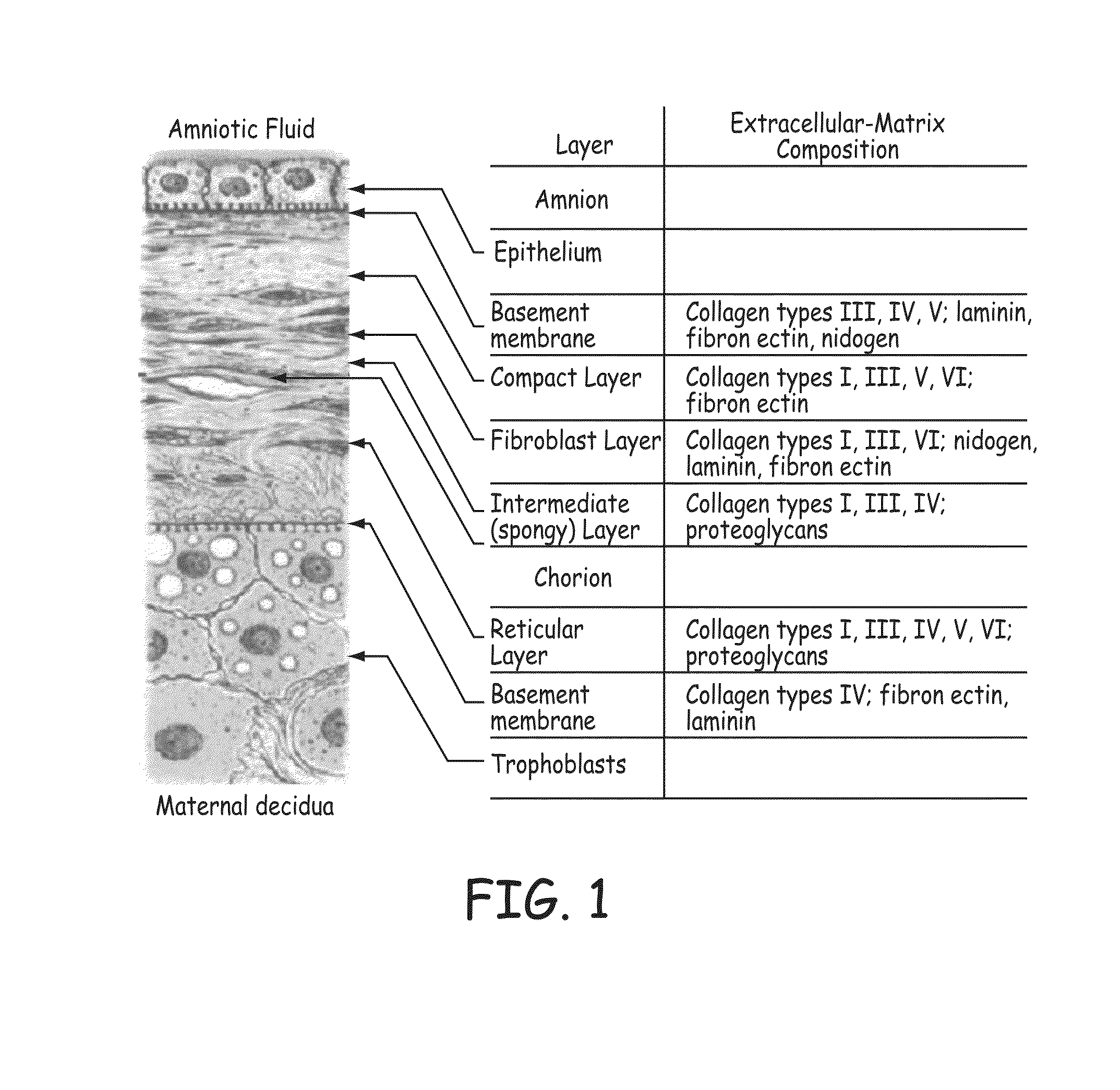
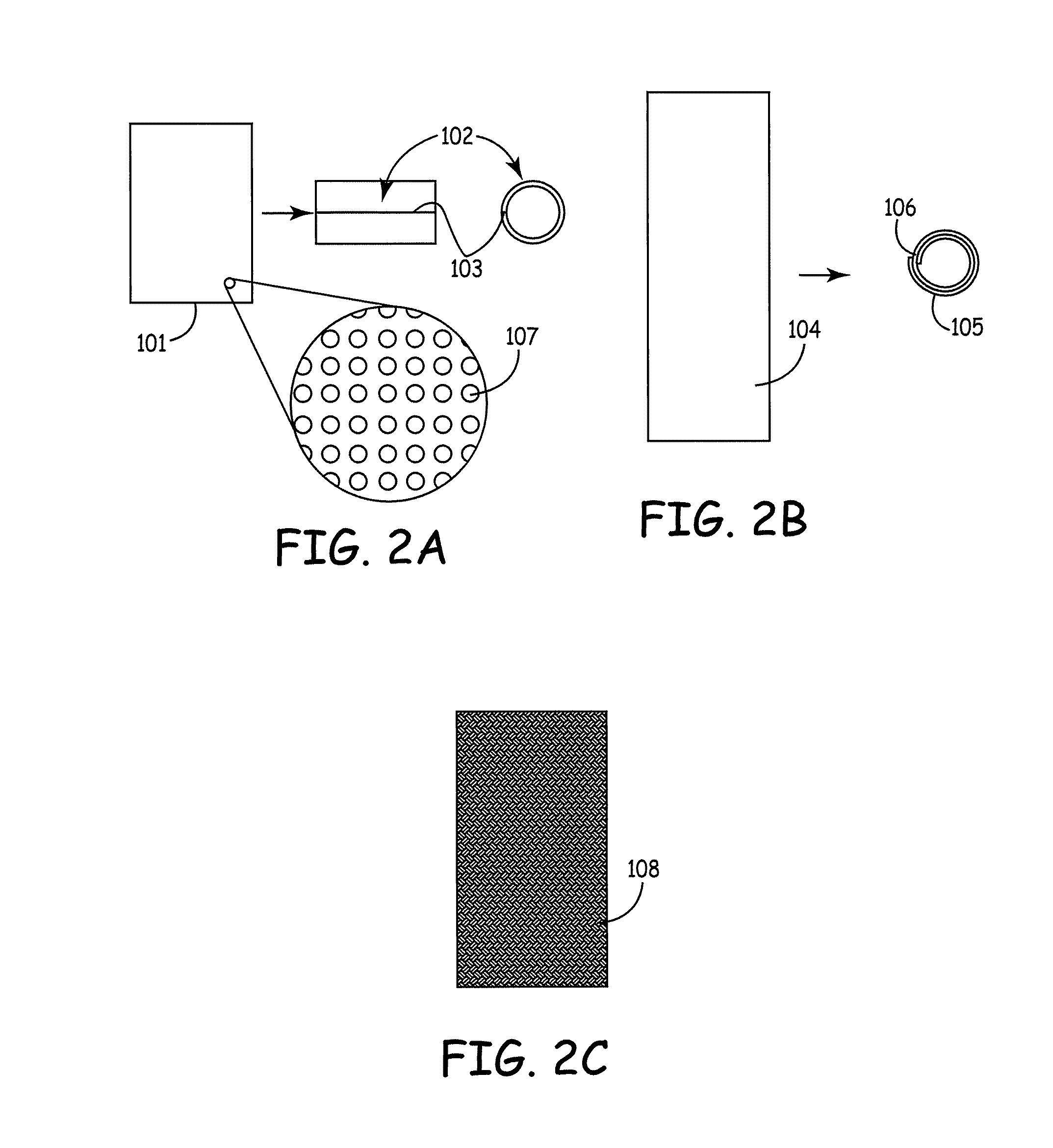
[0060]Stents described herein comprise a stent scaffold modified with an amnion tissue such that the stent provides an improved surface for contact with the patient's native tissues. A stent scaffold modification described herein provides a biocompatible stent with associated amnion tissue for use in arteries, e.g., coronary arteries, as well as for use in other lumens within the human body. Stent scaffolds can be modified, for example, with amnion tissue that is derived from amniotic sac of the placenta. Amnion tissue provides a surface with low or no antigenicity such that the tissue provides a uniquely appropriate material for the colonization of the material with native cells from the patient, such as endothelial cells. The stents generally are designed to facilitate the opening of a vessel lumen while providing for flow through the vessel, although other stent structures can be similarly modified. In some embodiments, the stents can have a generally cylindrical shape. The stent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com