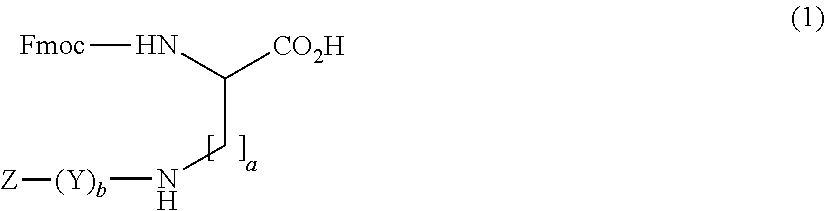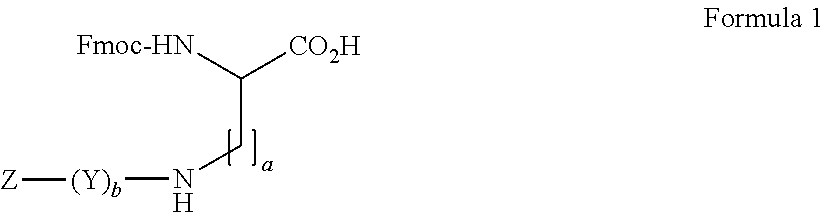Amino diacids containing peptide modifiers
a technology of amino diacids and modifiers, which is applied in the field of amino diacids containing peptide modifiers, can solve the problems of increasing the complexity of the modifiers, the clearance of native peptides or analogues thereof,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Method for the Synthesis of Side Chain Modified Diamino Acid Derivatives with the General Formula 1 in Aqueous Solution by the Acylation Fmoc-Lys-OH
[0271]To 18.4 g Fmoc-Lys-OH 200 ml Dioxan / 10%-NaHCO3(1:1) were added. The obtained mixture was then cooled to 0-5° C. and then equimolar amounts of Z—(Y)b—OH in 100 ml dioxan were added and the mixture was stirred for 2 h at 0-5° C. and 2 h at RT. The mixture was then diluted with 0.1 N—HCl and extracted with EtAc. The organic layer was then washed with 5%-NaHCO3, H2O, 0.1 N—HCl, H2O and brine, dryed over anhydrous Na2SO4 and concentrated in the RE. The obtained oily product precipitated by the addition of DEE or petroleum ether or water. The obtained solid was filtered and washed with DEE or PE or water and dried in vacuum. Yield 60-95%.
example 2
General Method for the Synthesis of Side Chain Modified Diamino Acid Derivatives with the General Formula 1 in Organic Solution by the Acylation of Fmoc-Lys-OH
[0272]To a suspension of 18.4 g Fmoc-Lys-OH in 200 ml DCM 5.4 ml Me3SiCl were added at 0° C. and stirred for 3 h. Then 12.9 ml DIPEA were added and stirred for additional 30 min. Then a solution of equimolar amounts of Z—(Y)b—OH, EDAC.HCl and HOSu in 100 ml anhydrous DMF were added and the mixture was stirred for 4 h at 10-15° C. The mixture was then diluted with 1 N—HCl and extracted with EtAc. The organic layer was then washed with 5%-NaHCO3, H2O, 0.1N—HCl, H2O and brine, dried over anhydrous Na2SO4 and concentrated in the RE. The obtained oily products precipitated by the addition of DEE or petroleum ether or water. The obtained solids were filtered washed with DEE and hexane and dried in vacuum. Yield: 65-95%.
example 3
Synthesis of N-Trityl-glutamic acid α-tert-butyl ester (Trt-Glu-OtBu). Formula Nr. 2′-1
[0273]
[0274]40.6 g H-Glu-(OtBu) were suspended in 400 ml DCM and cooled to 0° C. Then 21.7 g chlorotrimethylsilane were added dropwise and the mixture was stirred until a clear solution was obtained. Then 52 g DIPEA were added followed by 56 g Trt-Cl and the mixture was stirred for additional 2 h at 0° C. and warmed up to RT and stirred farther for additional 2 h. Then 20 ml MeOH were added and the mixture was concentrated in vacuum and then 500 ml DEE were added and the product was extracted and purified by acidic-basic extraction. The organic solution was concentrated in vacuum and the Trt-Glu-OtBu was obtained as syrup. Yield: 86.0 g (76.7%). The obtained syrup can be converted to solid diethylammonium salt by dissolving it in 350 ml DEE and adding to the solution 15 g DEA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com