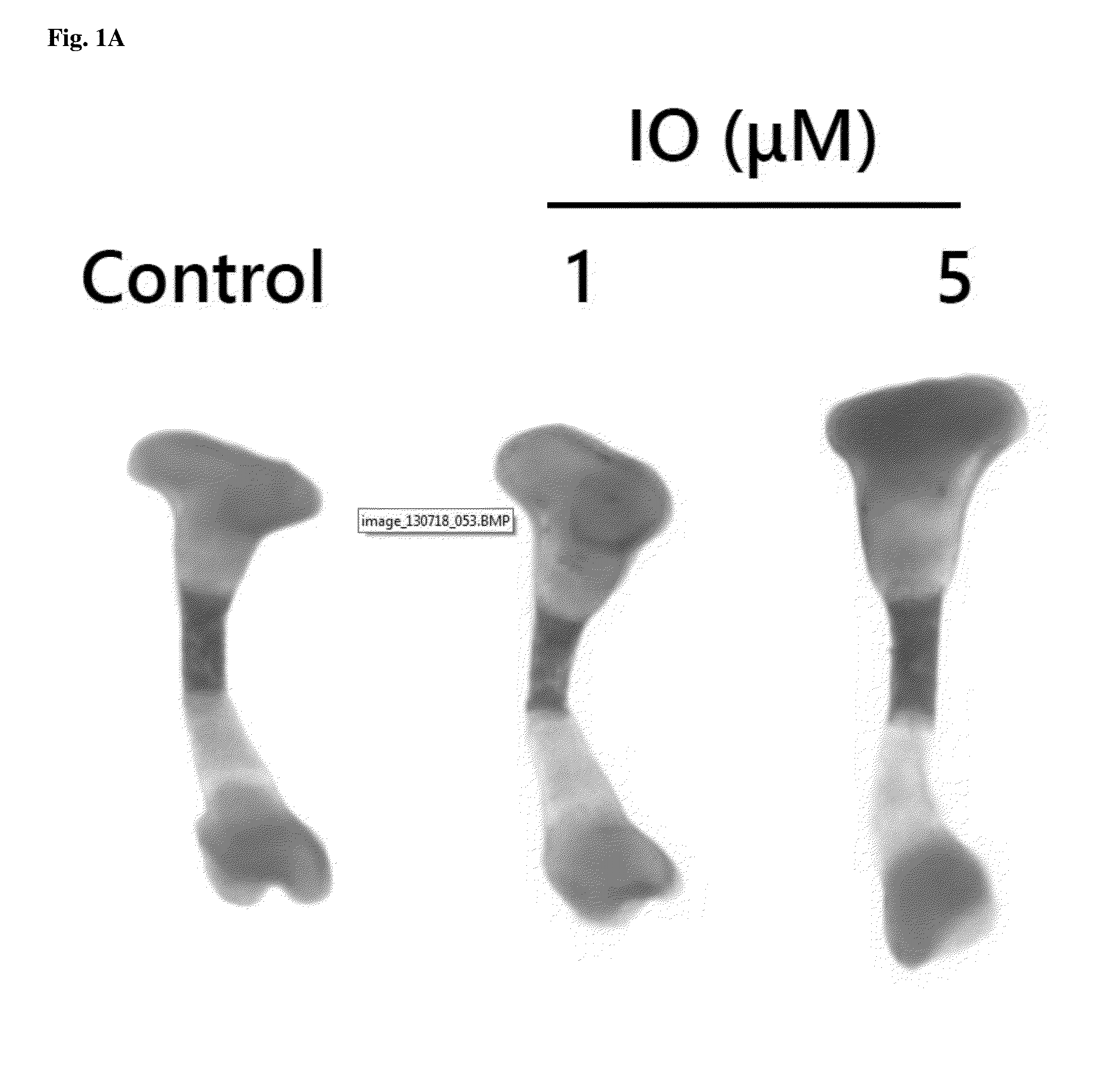
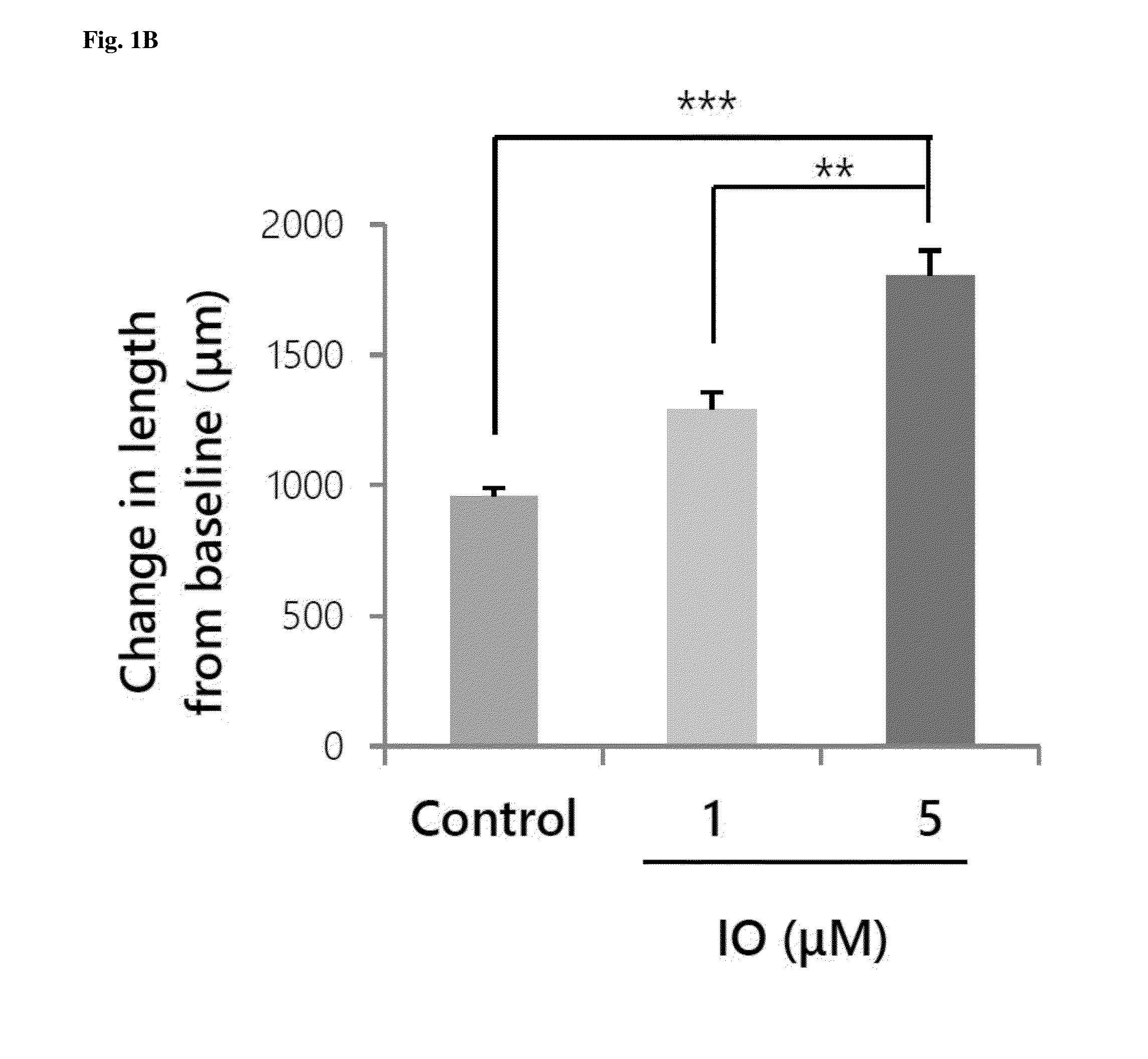
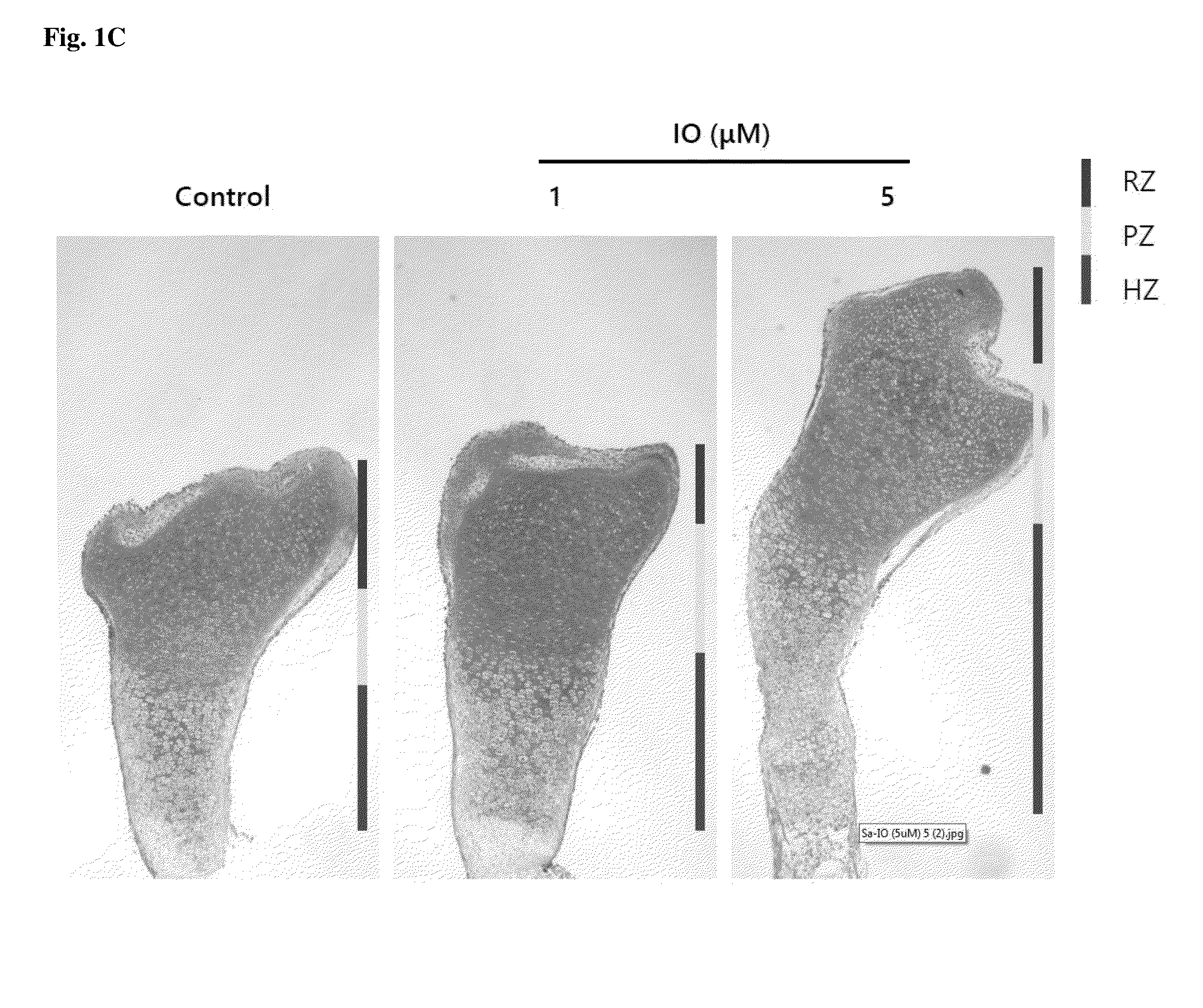
Small molecule, indirubin-3-oxime, for prevention and treatment of bone disease
a small molecule, bone disease technology, applied in the direction of skeletal disorder, food preparation, medical preparation, etc., can solve the problems of inconvenient use, no drug has a direct target for height growth, and growth hormone injection is not suitable for a child with normal hormone secretion, etc., to achieve low toxicity, low development cost, and clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cell Culture and Reagents
[0026]Rat chondrosarcoma (RCS) cells were cultured in a Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, N.Y.), supplemented with 10% fetal bovine serum (FBS) and penicillin / streptomycin.
example 2
Western Blot Analysis
[0027]For Western blot analysis, cells were treated with indirubin-3′-oxime for 24 hours, cultured and reached to 70% confluency and then were harvested. After the harvested samples underwent cytolysis, the proteins were quantified, which was followed by electrophoresis using 10% or 12% SDA-PAGE gel. Afterwards, immunoblot was conducted using antibodies.
example 3
[0028]2.5×104 cells were adhered to a cover glass placed in a 12-well plate. Once securely attached, cells were treated with indirubin-3′-oxime for 24 hours, and then were rinsed with a phosphoric acid buffer solution. After adding 4% paraformaldehyde, cells were fixed at room temperature for 20 min. Then, proteins expressed in the cells were observed through cell membrane permeation, blocking, and immunofluorescent staining processes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com