Cardiomyocytes From Induced Pluripotent Stem Cells From Patients and Methods of Use Thereof
a technology of cardiomyocytes, which is applied in the field of patient-induced pluripotent stem cells of cardiomyocytes, can solve the problems of undermining the value of these methodologies for understanding the cellular and physiological processes of dcm, disrupting the normal alignment of cardiomyocytes, and affecting the function of patients, so as to reduce the beating rate, improve the function, and compromise the effect of contraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
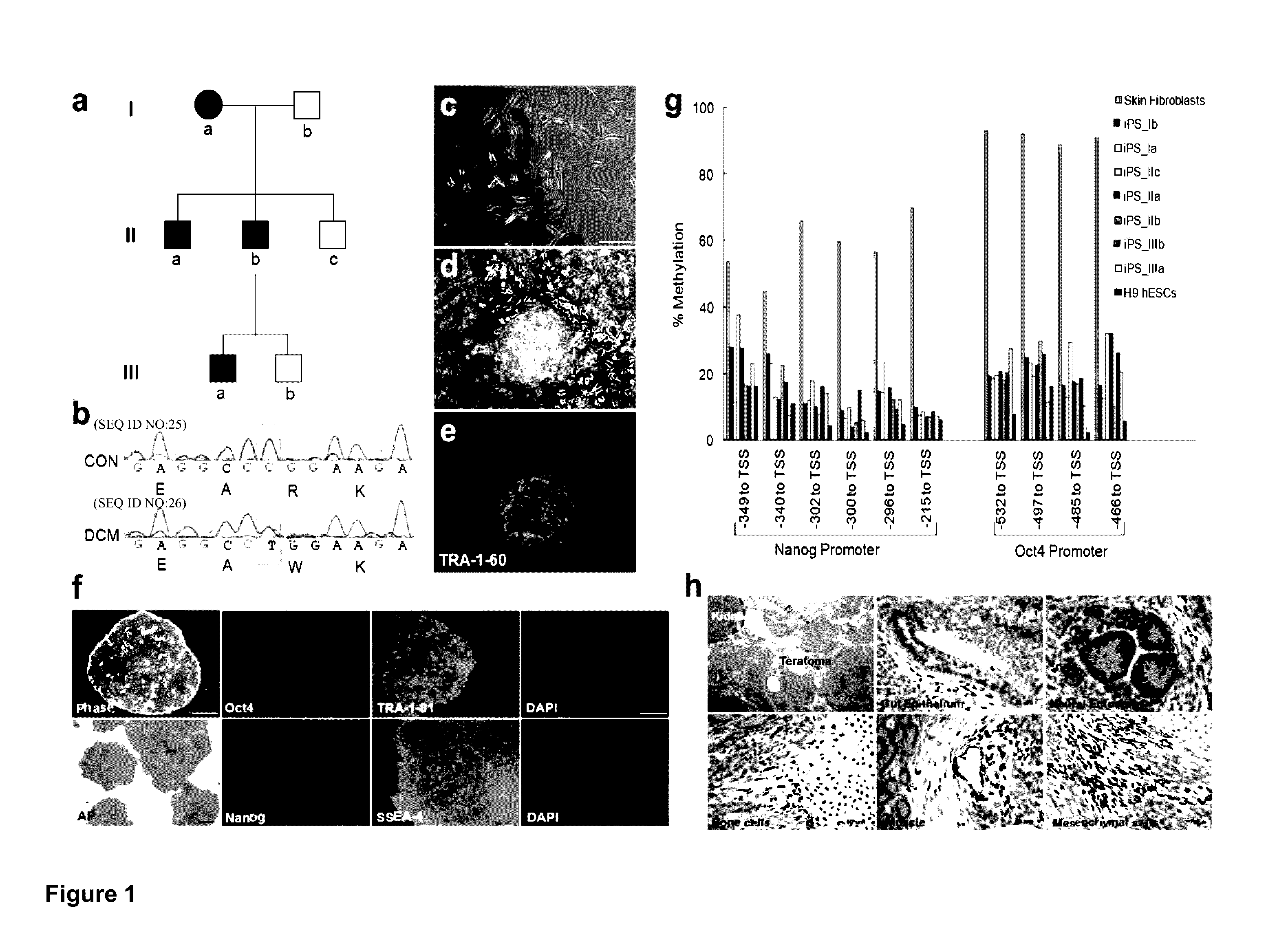
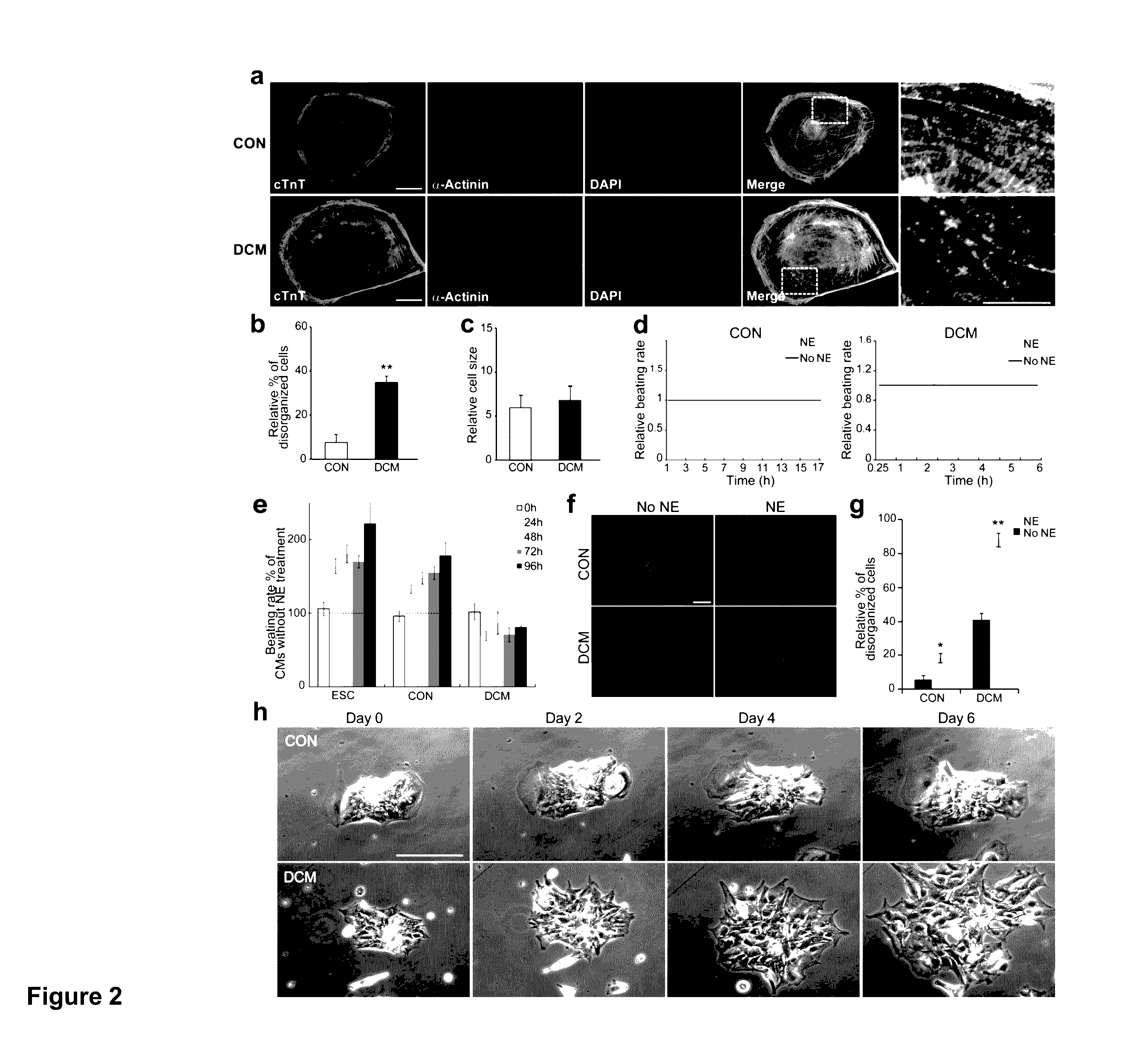
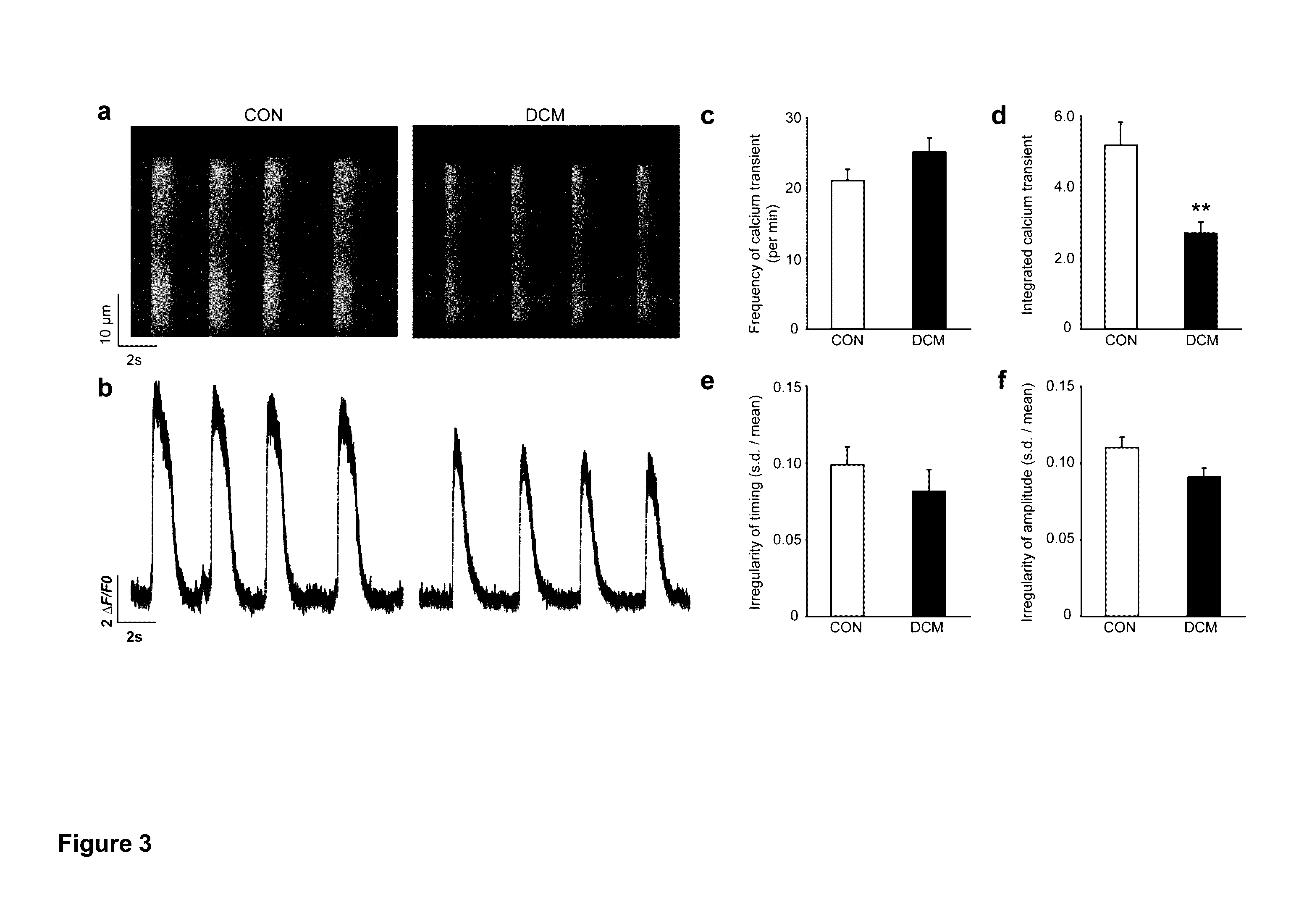
[0117]Dilated cardiomyopathy (DCM) is the most common cardiomyopathy, characterized by ventricular dilatation, systolic dysfunction, and progressive heart failure. DCM is the most common diagnosis leading to heart transplantation and places a considerable burden on healthcare worldwide. Here we generated cardiomyocytes (CMs) from iPSCs derived from patients of a DCM family carrying a point mutation (R173W) in the gene that encodes sarcomeric protein cardiac troponin T. Compared to the control healthy individuals in the same family cohort, DCM iPSC-CMs exhibited decreased calcium transient amplitude, decreased contractility, and abnormal sarcomeric α-actinin distribution. When stimulated with β-adrenergic agonist, DCM iPSC-CMs showed characteristics of failure such as reduced beating rates, compromised contraction, and significantly more cells with abnormal sarcomeric α-actinin distribution. β-adrenergic blocker treatment and over-expression of sarcoplasmic reticulum Ca2+ ATPase (Ser...
example 2
Cardiomyocytes from Patients with Hypertrophic Cardiomyopathy
[0147]Hypertrophic cardiomyopathy (HCM) is an autosomal dominant disease of the cardiac sarcomere, and is estimated to be the most prevalent hereditary heart condition in the world. Patients with HCM exhibit abnormal thickening of the left ventricular (LV) myocardium in the absence of increased hemodynamic burden and are at heightened risk for clinical complications such as progressive heart failure, arrhythmia, and sudden cardiac death (SCD). Molecular genetic studies from the past two decades have demonstrated that HCM is caused by mutations in genes encoding for proteins in the cardiac sarcomere. While identification of specific mutations has defined the genetic causes of HCM, the pathways by which sarcomeric mutations lead to myocyte hypertrophy and ventricular arrhythmia are not well understood. Efforts to elucidate the mechanisms underlying development of HCM have yielded conflicting results, paradoxically supporting...
example 3
Cardiomyocytes from Patients with Anthracycline Toxicity
[0187]Anthracycline-induced cardiotoxicity (and resistance to anthracycline-induced toxicity). Anthracyclines such as doxorubicin are frontline chemotherapeutic agents that are used to treat leukemias, Hodgkin's lymphoma, and solid tumors of the breast, bladder, stomach, lung, ovaries, thyroid, and muscle, among other organs. The primary side effect of anthracyclines is cardiotoxicity, which results in severe heart failure for many of the recipients receiving regimens utilizing this chemotherapeutic agent. Patient specific iPSC-cardiomyocytes (iPSC-CMs) were derived from individuals who are susceptible to anthracycline-induced cardiotoxicity as well as from individuals who are not susceptible to anthracycline-induced cardiotoxicity.
[0188]These cells are useful to detect and titrate cardiotoxic chemotherapeutic drugs, as well as identify genes responsible for susceptibility / resistance to anthracycline-induced cardiotoxicity. Age...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com