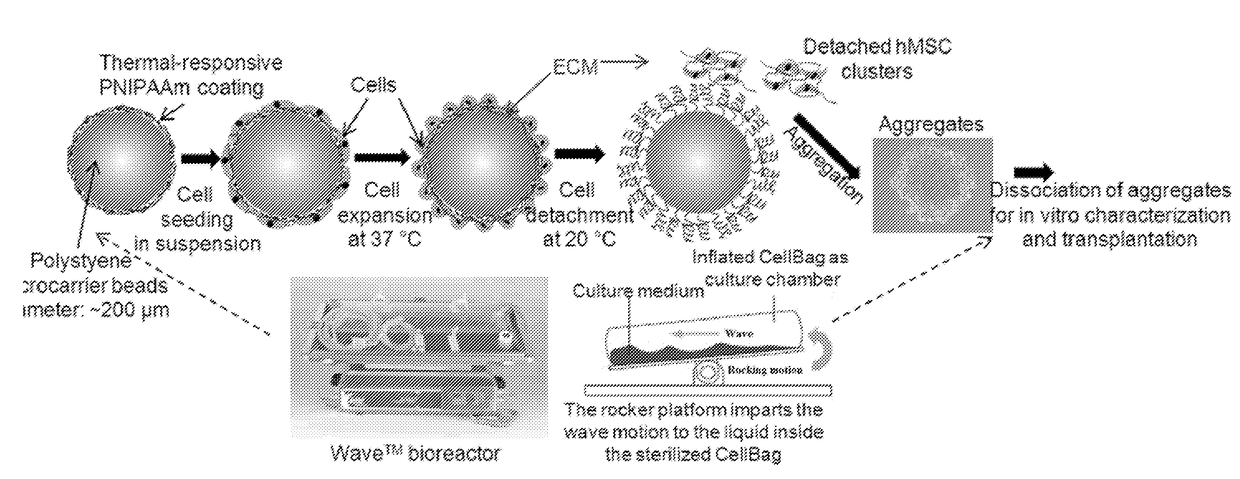
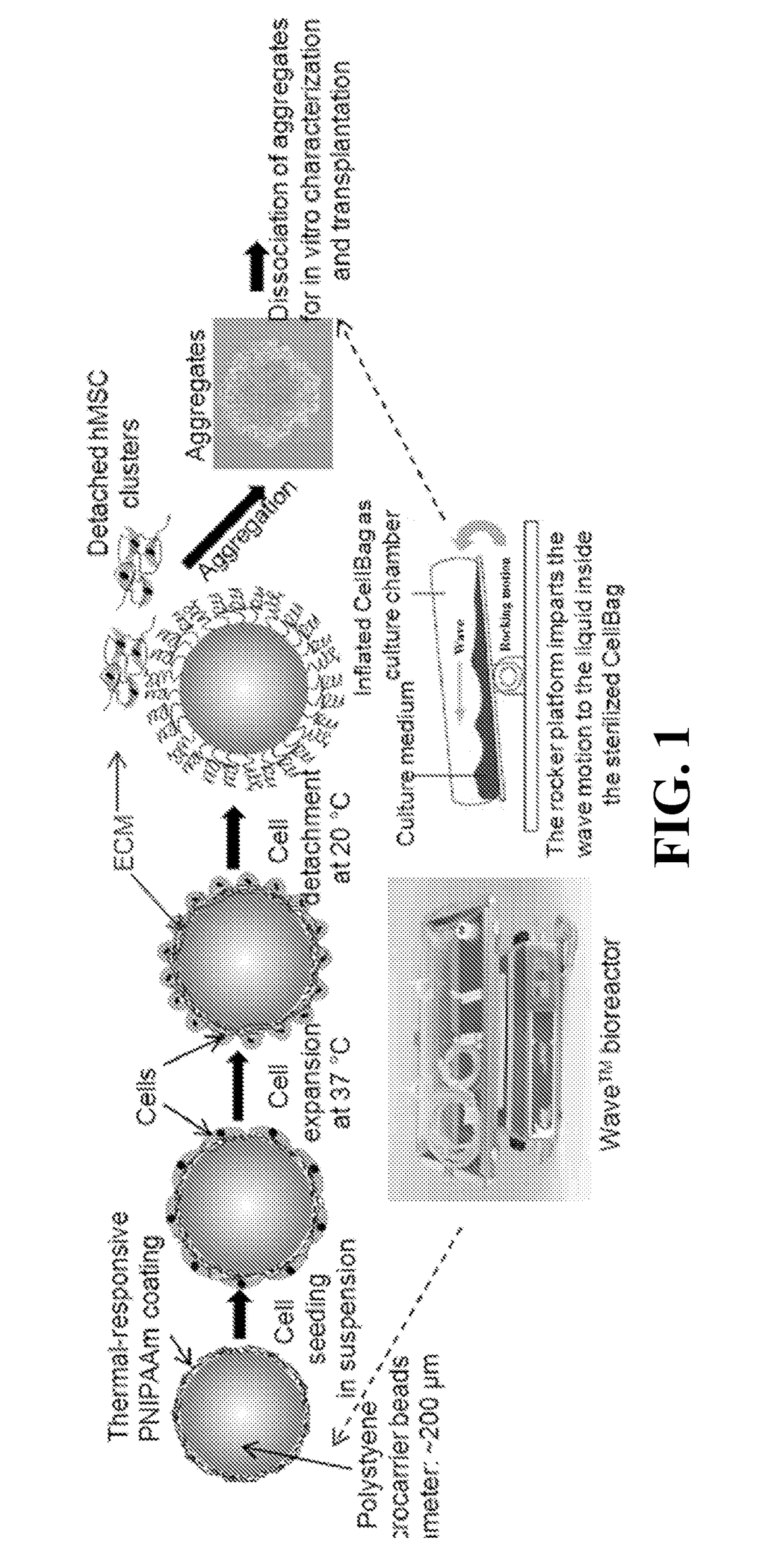
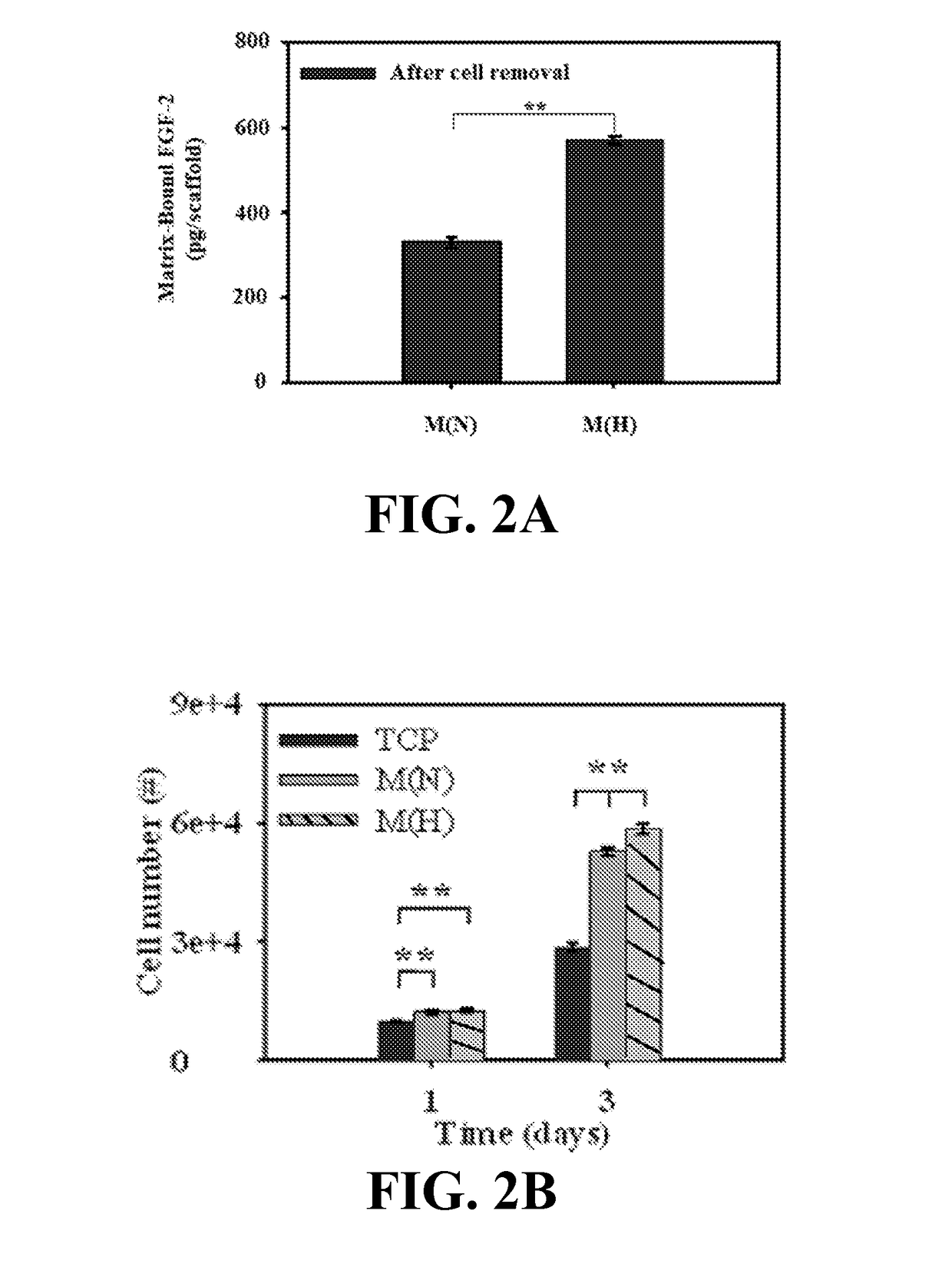
Materials and methods for expansion of stem cells
a technology of stem cells and materials, applied in the field of stem cell expansion materials and methods, can solve the problems of reducing the therapeutic potency of hmsc transplantation, cell senescence, and low engraftment and homing efficiency of culture-expanded hmsc transplantation, so as to improve or enhance the therapeutic potency of cells, improve the translational success of cells, and enhance the therapeutic effect of stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Actin-Mediated Contractility Influences the Assembly of Multicellular hMSC Aggregates
[0076]While the initial steps of aggregation require close contact and cell-cell adhesion via cadherin molecules, reorganization of actin cortical network is crucial in the establishment of a mature cell-cell contact (Amack and Manning 2012). To investigate the temporal effects of actin-mediated contractility in hMSC aggregate formation, hMSC cultures were treated with cytoD (1) in plastic culture for 2 days prior to cell detachment (e.g., 2D pretreatment) or (2) 12 hours after aggregate formation on ULA surfaces (i.e., 3D treatment). In 2D pretreatment, hMSC displayed dose-dependent response in which cytoD disrupted hMSC aggregation at concentration above 0.6 μM and prevented aggregate compaction at lower concentrations (FIGS. 5A and 5B). In 3D treatment, hMSC aggregates remained intact in cytoD concentration of 0.6 μM and exhibited dose-dependent reduction on compaction similar to those of 2D pret...
example 2
Actin Mediates Aggregate Compaction but not Viability and Caspase Expression
[0078]LPA is a naturally occurring bioactive phospholipid with multiple biological functions that include its ability to initiate cytoskeleton contraction by RhoA activation, promote cell survival and proliferation, and to enhance survival of hypoxia-challenged neonatal cardiomyocytes (Tigyi et al. 1994; Moolenaar 1995). LPA has also been identified as a novel survival factor and protects MSCs against hypoxia and serum deprivation-induced apoptosis (Chen et al. 2008a). Treatment of hMSC aggregates by LPA, however, has limited effects on aggregate compaction as well as viability at LPA concentration up to 10 μM (FIGS. 7A-7E).
[0079]The involvement of actin-myosin based contractility was investigated by treatment of aggregates with Y-27632, which inhibits the phosphorylation of Rho-associated kinase (ROCK) and prevents cell compaction (Chen et al. 2010). As expected, inhibition of ROCK kinase by 10 μM Y-27632 r...
example 3
Actin Mediates Aggregate Morphology, Interaction, and Spreading on Adherent Surfaces
[0082]Disruption of actin significantly alters hMSC morphology in the aggregates. As shown in FIGS. 10A-1, 10A-2, 10B and 10C, cells in the hMSC aggregates were tightly packed and spread with limited interstitial space at the boundary of the aggregate. In contrast, cells in the cytoD- and Y-27632-treated aggregates were loosely packed and exhibited spherical morphology with abundant interstitial space as shown by SEM and histology (FIGS. 10A-1 and 10A-2). Histological sectioning also revealed contrasting morphology of hMSC in the interior of control and cytoD and Y-27632-treated aggregates. In the control aggregates, hMSCs are morphologically heterogeneous with spindle-shaped cells at the outer boundary and round and tightly packed cells in the interior, indicating morphological polarization. In the cytoD and Y-27632 treated aggregates, cells are loosely packed with no spreading at the boundary, indi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com