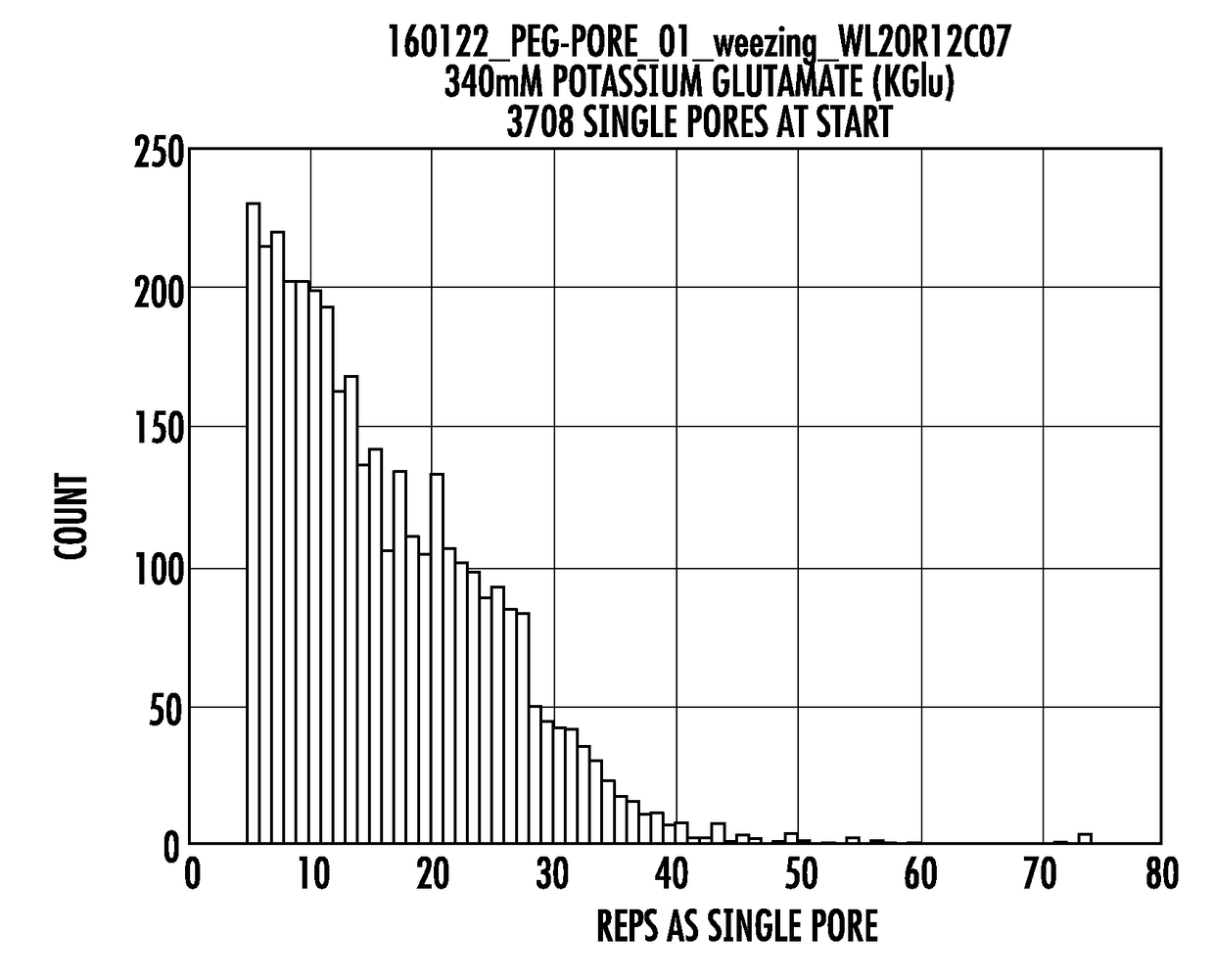
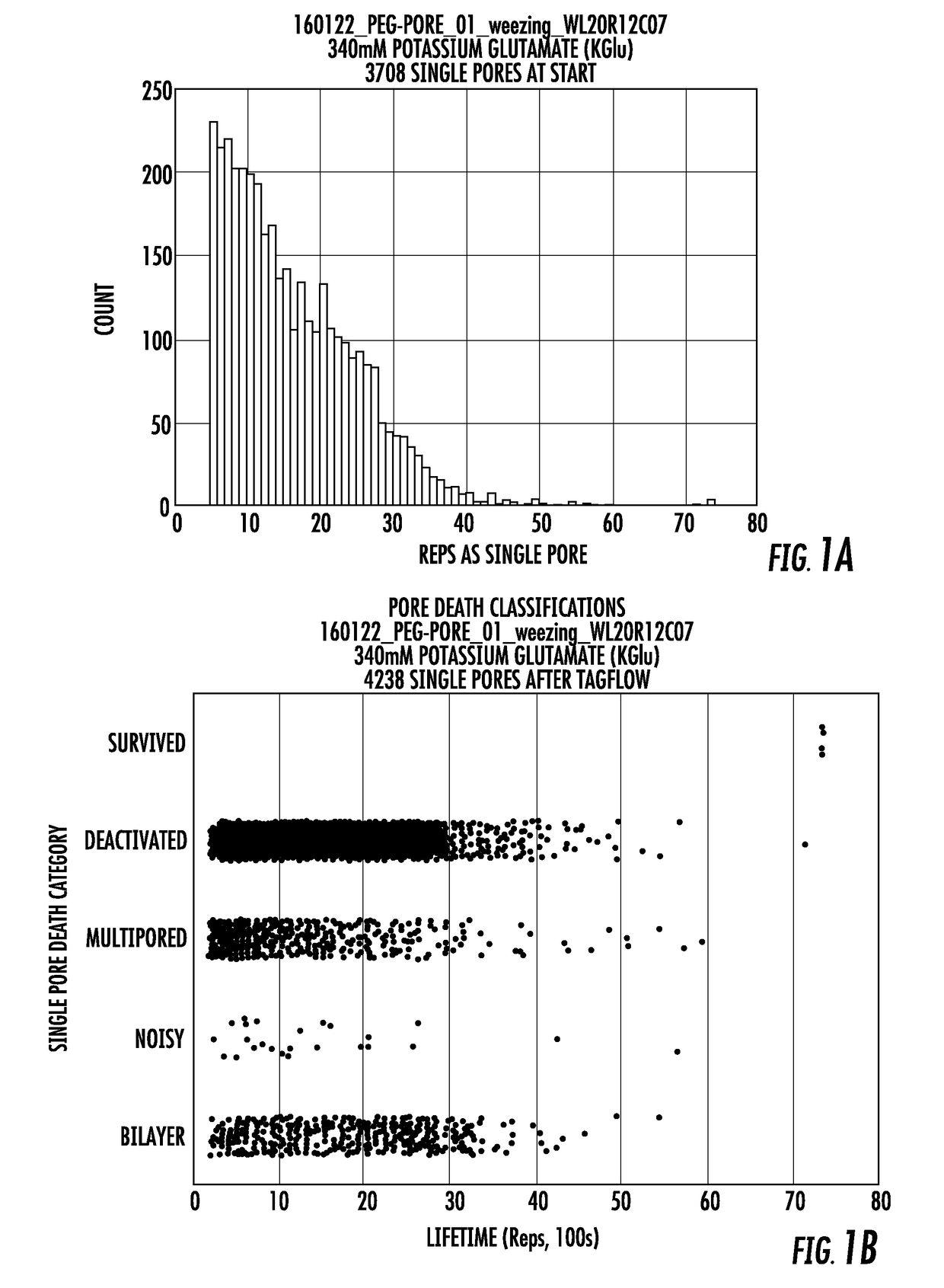
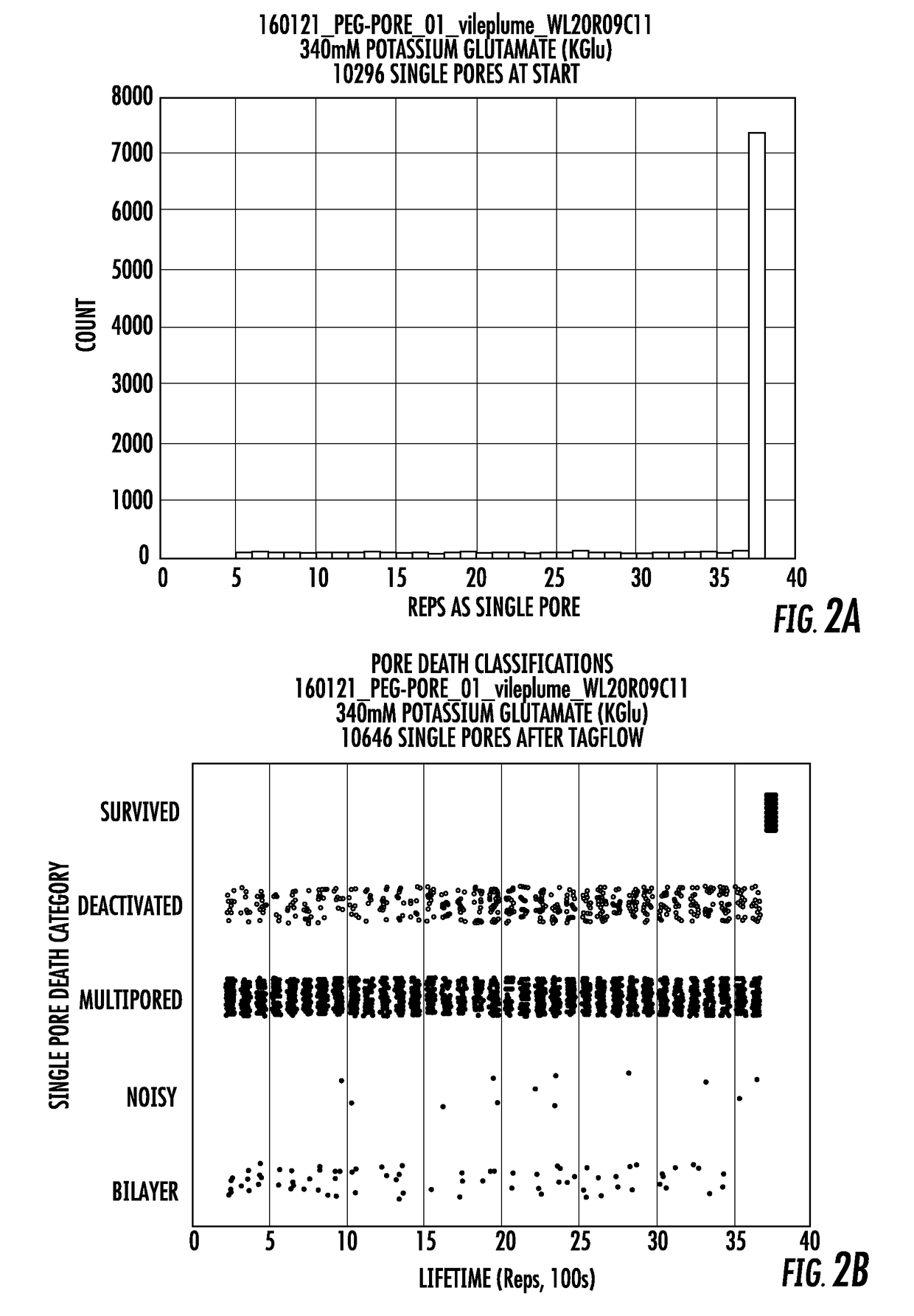
Long lifetime alpha-hemolysin nanopores
a nanopore and alpha-hemolysin technology, applied in the field of alpha-hemolysin variants of staphylococcus aureaus, can solve the problems of difficult task, short time-consuming and laborious nanopores using wild-type alpha-hemolysins, and often serve as rate-limiting features of alpha-hemolysin nanopores during sequencing reactions, etc., to achieve more sequencing data than the effect of improving the lifetime of the nanopor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression and Recovery
[0136]This example illustrates the expression and recovery of protein from bacterial host cells, e.g., E. coli.
[0137]DNA encoding the wild-type a-HL was purchased from a commercial source. The sequence was verified by sequencing.
[0138]Plasmid Construction.
[0139]The gene encoding either a wild-type or variant α-hemolysin was inserted into a pPR-IBA2 plasmid (IBA Life Sciences, Germany) under the control of T7 promoter.
[0140]Transformation.
[0141]E. coli BL21 DE3 (from Life Technologies) cells were transformed with the expression vector comprising the DNA encoding the wild-type or variant α-hemolysin using techniques well-known in the art. Briefly, the cells were thawed on ice (if frozen). Next, the desired DNA (in a suitable vector / plasmid) was added directly into the competent cells (should not exceed 5% of that of the competent cells) and mixed by flicking the tube. The tubes were placed on ice for 20 minutes. Next, the cells were placed in a 42° C. water bat...
example 2
Alpha-Hemolysin Variants
[0147]The following example details the introduction of a mutation at a desired residue.
[0148]Mutations.
[0149]Site-directed mutagenesis is carried out using a QuikChange Multi Site-Directed Mutagenesis kit (Stratagene, La Jolla, Calif.) to prepare the example H35G+V149K+H144A (SEQ ID NO: 4) and H35G+E111N+M113A+126−131G+H144A+K147N (SEQ ID NO: 19), with the sequences including a C-terminal linker / TEV / HisTag for purification. QuikChange Multi Site-Directed Mutagenesis kit (Stratagene, La Jolla, Calif.) is also carried out to prepare a variant (E111N+M113A+126−131G+K147N, SEQ ID NO: 20) for Polymerase attachment, with the variant including a C-terminal SpyTag, KG linker, and HisTag. The variants were expressed and purified as in Example 1.
example 3
Assembly of Nanopore Including Variants
[0150]This example describes the assembly of a 1:6 heptameric nanopore including one subunit having a SpyTag sequence for subsequent polymerase attachment (the “α-HL-variant-SpyTag” subunit) and six α-HL-variant subunits with no SpyTag (the “α-HL-variant” subunits).
[0151]The α-HL-variant-SpyTag (E111N+M113A+126−131G+K147N, SEQ ID NO: 20)) was prepared and expressed as described in Examples 1 and 2 with a C-terminal SpyTag, KG linker, and HisTag. The α-HL-variant-SpyTag protein was then then purified on a cobalt affinity column using a cobalt elution buffer (200 mM NaCl, 300 mM imidazole, 50 mM tris, pH 8). The protein was stored at 4° C. if used within 5 days, otherwise 8% trehalose was added and stored at −80° C.
[0152]For the α-HL-variant subunits, variants of H35G+E111N+M113A+126-131G+H144A+K147N (SEQ ID NO: 19) were prepared and expressed as described in Examples 1 and 2 with a linker / TEV / HisTag and purified on a cobalt affinity column using...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| α | aaaaa | aaaaa |
| lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com