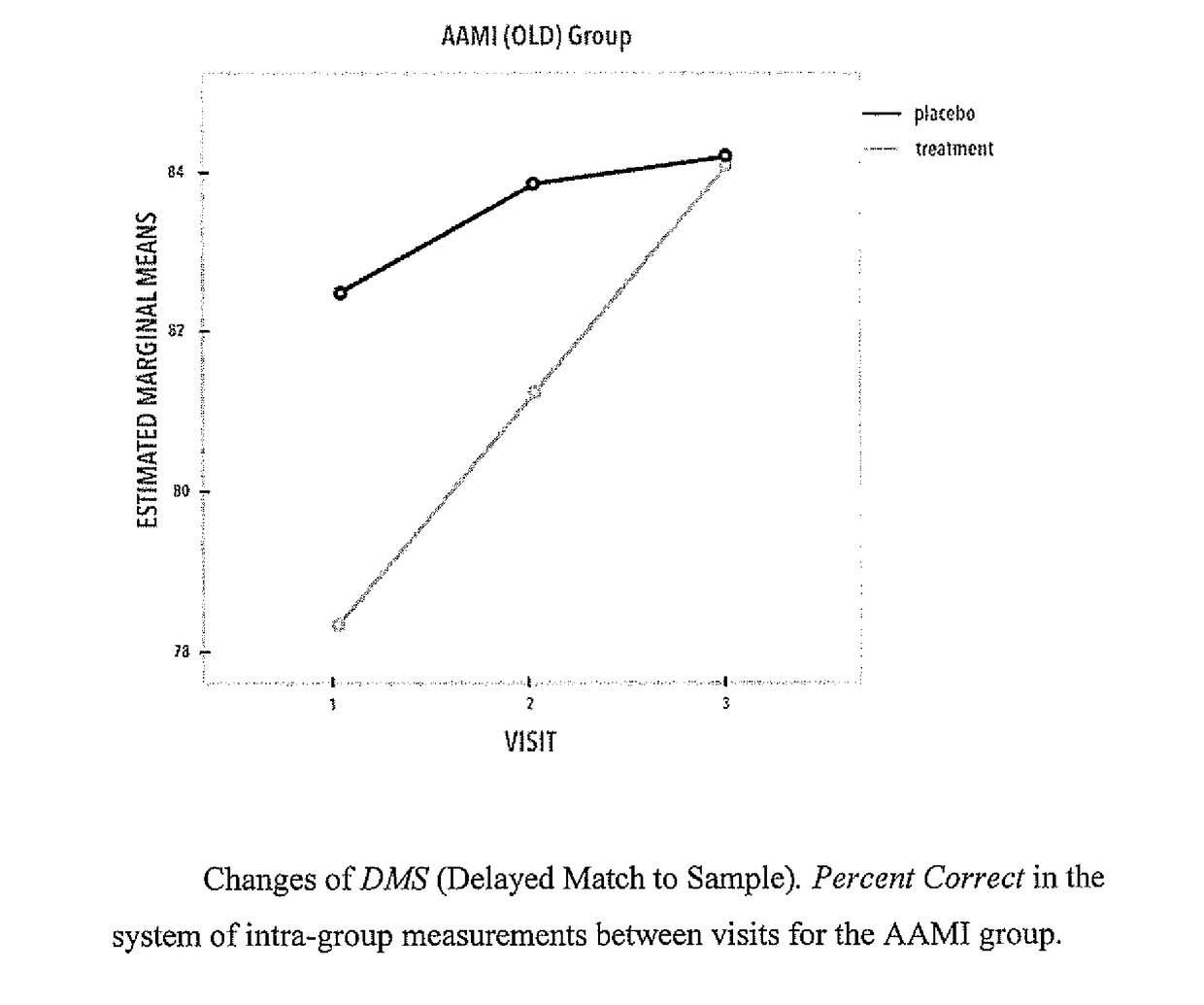
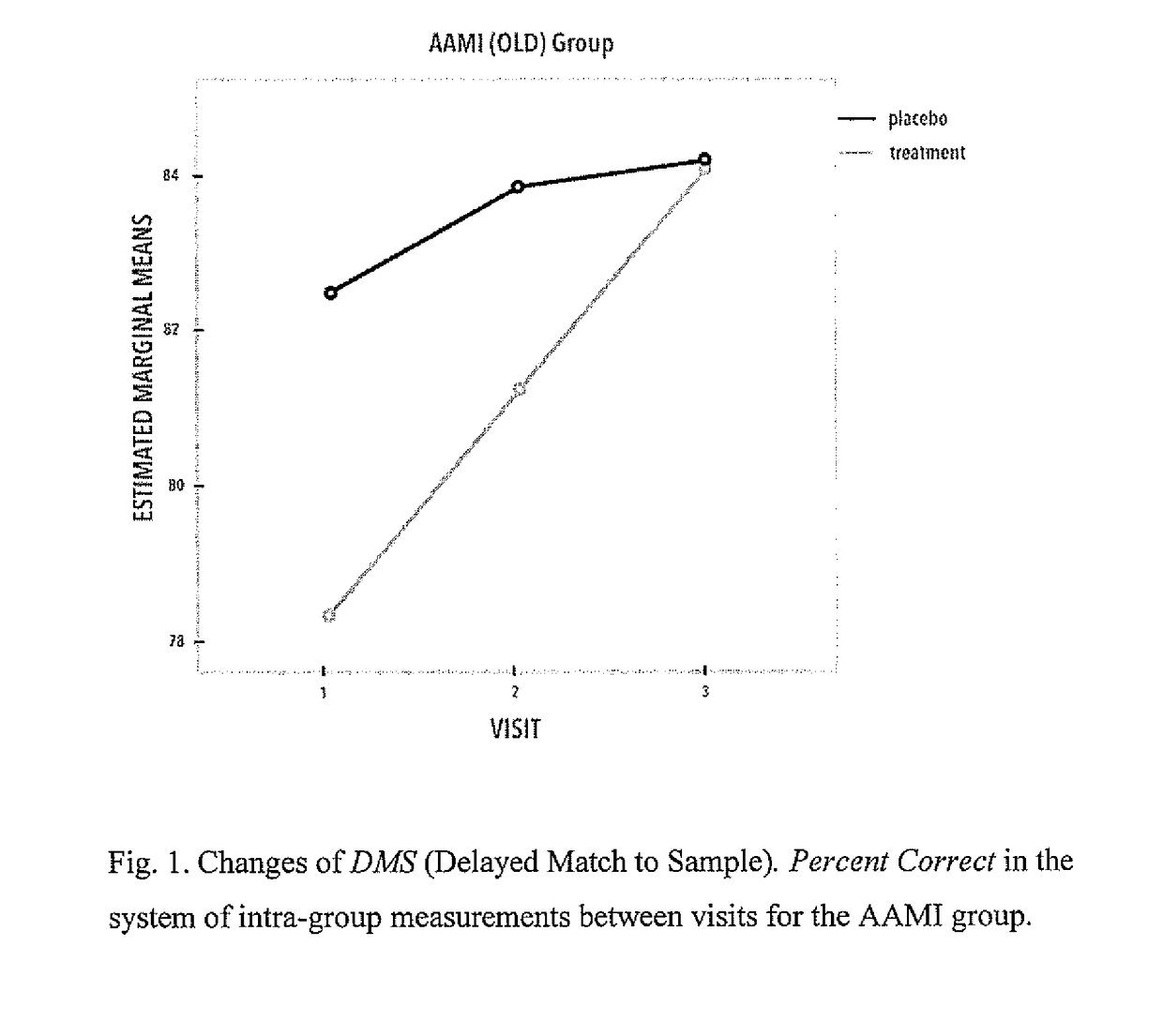
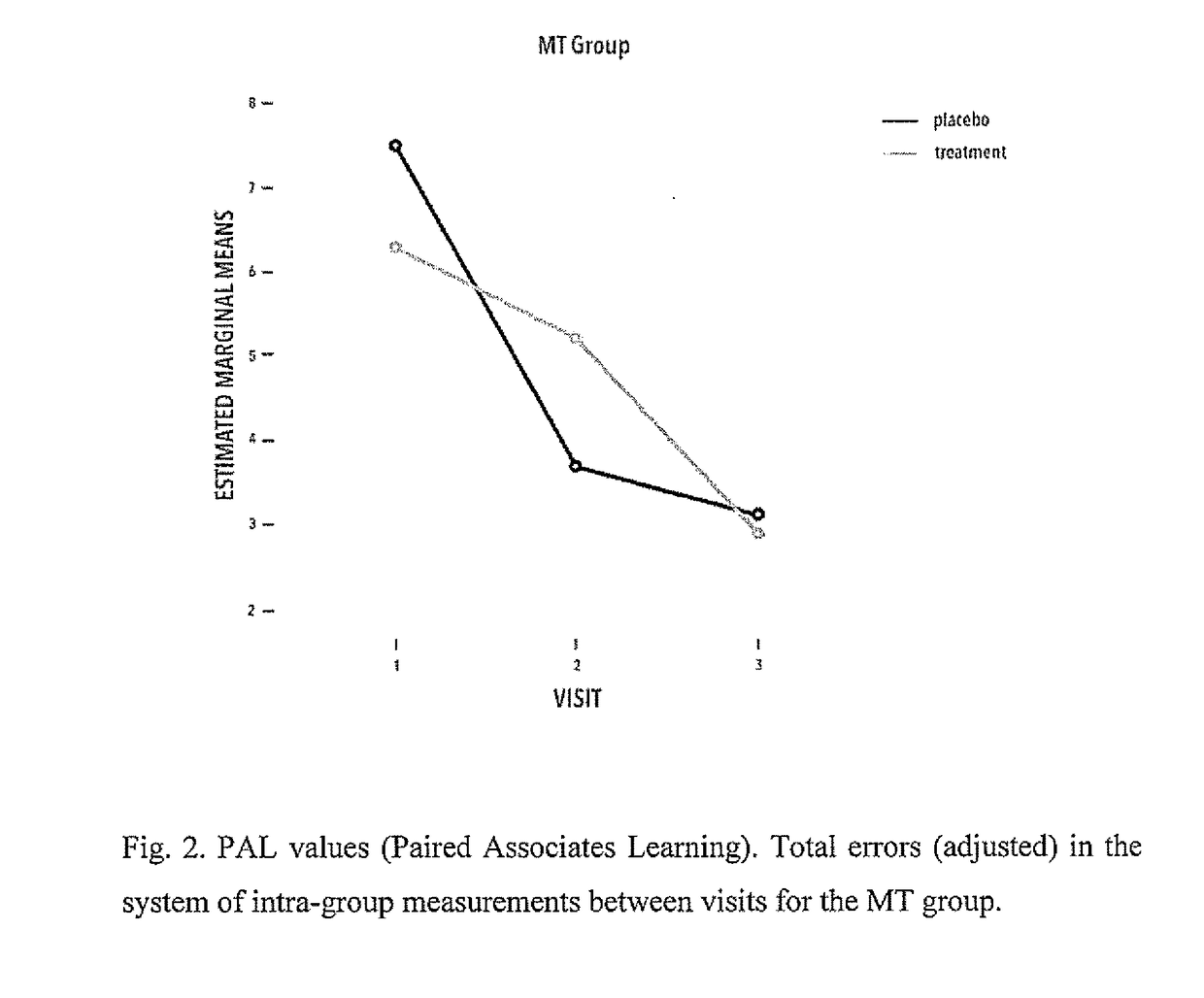
Proline-rich polypeptide complex for use in treatment of bdnf-dependent disorders
a polypeptide complex and bdnf-dependent technology, applied in the field of proline-rich polypeptide complexes, can solve the problems of unknown mechanism underlying the alleged neuroprotective effect of prps in alzheimer's disease, and achieve the effects of supporting the development of the nervous system, increasing the bdnf level in the blood, and activating immunomodulatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pharmaceutical Composition (Tablets):
[0111]
IngredientmgCOLOCO prp0.12Lactose monohydrate DC100.00Lactose 200 mesh70.86Sorbitol270.00Microcrystalline cellulose69.00Xanthan gum6.00Vanilla flavour6.00Silicon dioxide3.00Magnesium stearate5.00Total530.00
Total weight of a tablet containing the following ingredients is 530 mg.
Infant Milk Formula Stage 1 Enriched with COLOCO Prp (GM-1C)
Formula milk stage 1 is an infant formula for babies 0-5 months, commercially available MAMI LAC 1, supplemented with COLOCO prp in the amount of 20 μg per 100 mg of the milk formula powder, providing the dose of COLOCO prp 2.7 μg per 100 ml of the ready to drink product.
Per 100 ml of readyCompositionto drink product100 gEnergykcal69510kJ2902140Fat:g3.525.5Linoleic acidmg4503300α-linolenic acidmg61450Protein:g1.289.5Caseing0.513.8Whey-proteing0.775.7Carbohydrates:g8.260.6Lactoseg7.454.8Maltodextring0.85.8Moisture Vitamins:g3.0Vit. Aμg85630Vit. D3μg1.410.5Vit. Emg1.18.1Vit. K1μg4.130Vit. Cmg8.160Vit. B1μg58430...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| obesity-related | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com