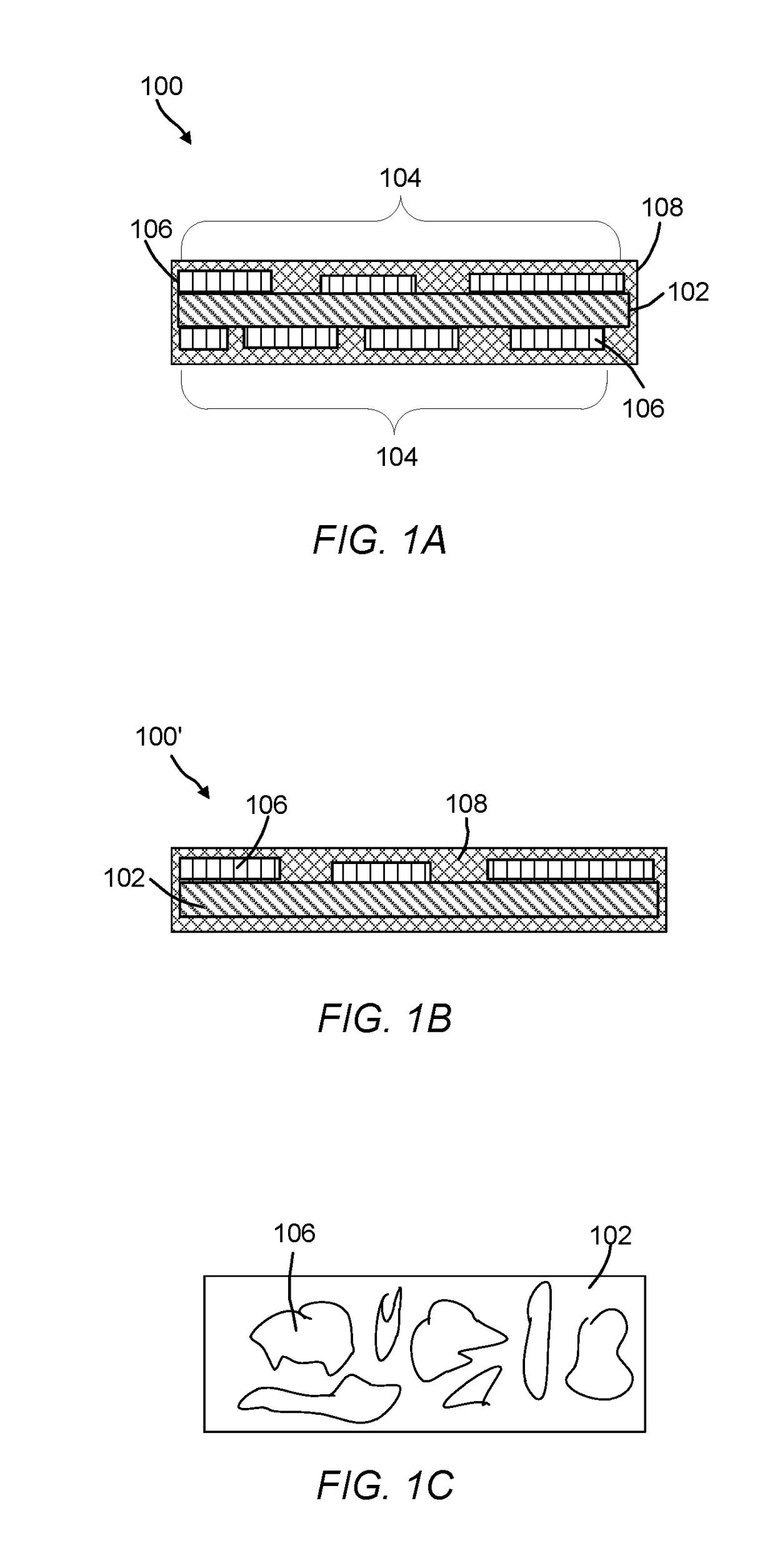
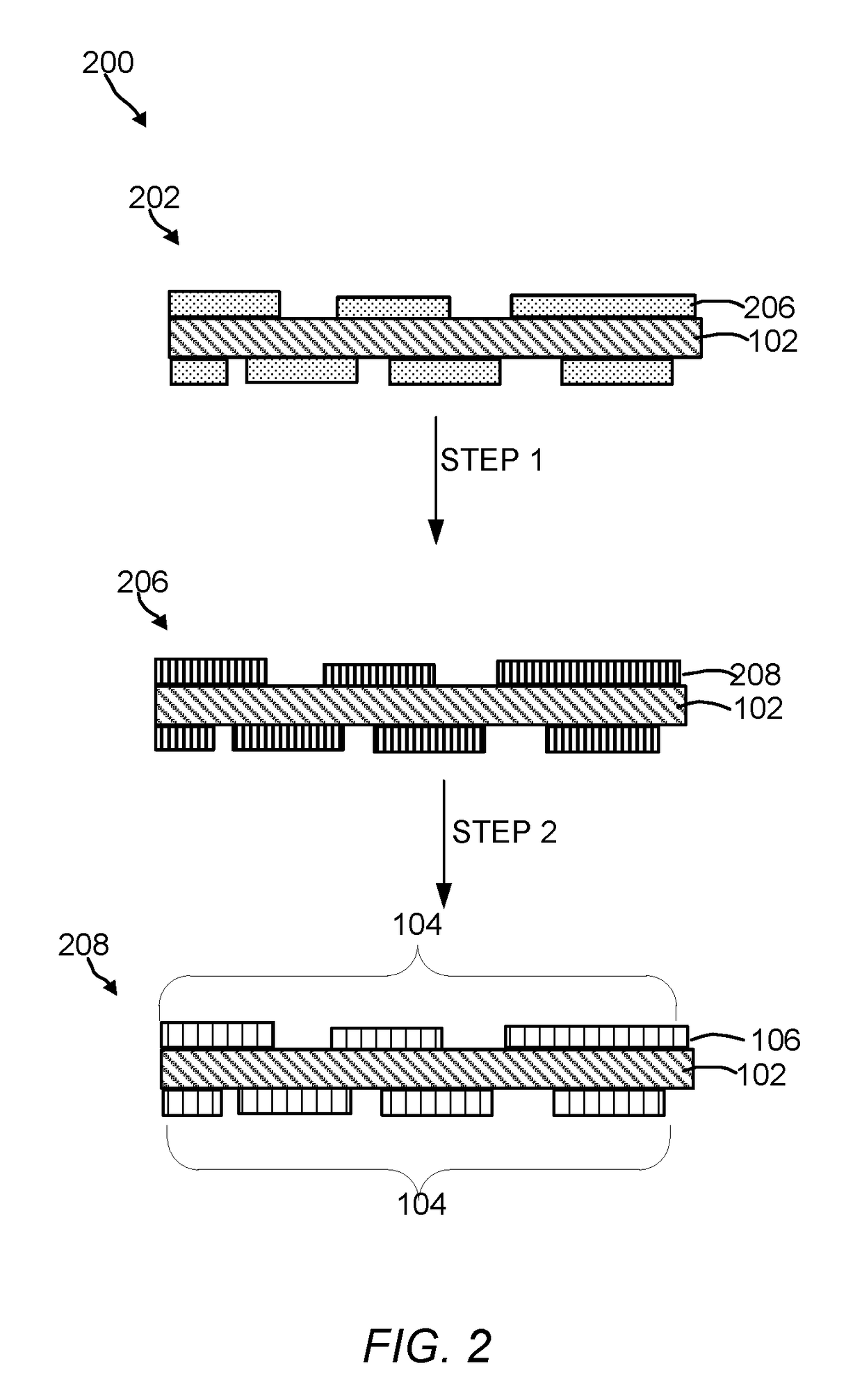
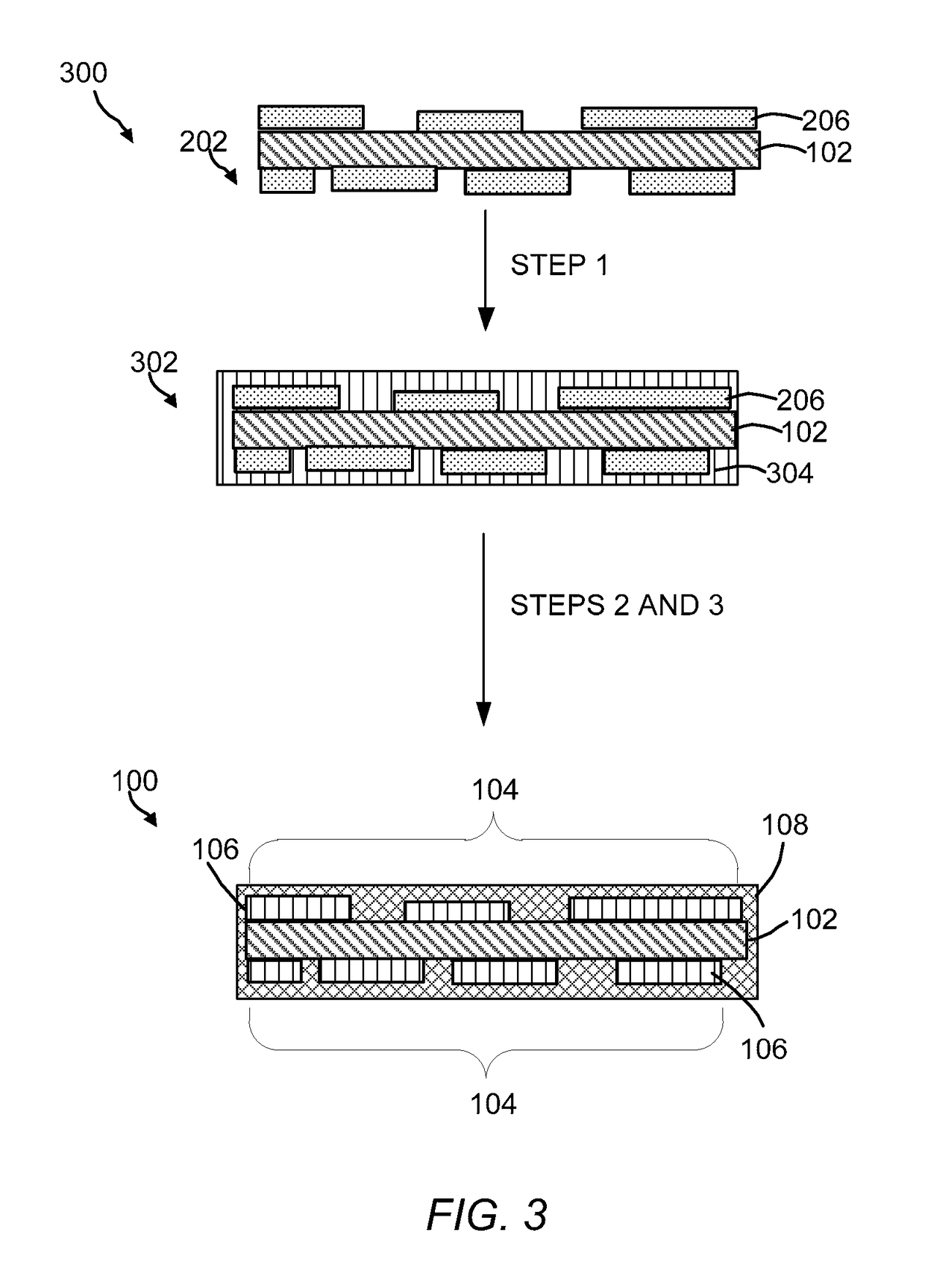
Oxygen evolution electrocatalysts with carbon coated cobalt (ii, iii) oxide layers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of OER Electrocatalyst of the Present Invention
[0071]Materials:
[0072]All chemical reagents including cobalt(II) nitrate hexahydrate, glucose, potassium hydroxide (KOH), sulfuric acid (H2SO4) and ethanol were purchased from Sigma Aldrich® (U.S.A.). Ultrapure water was obtained from a Millipore filtration system.
[0073]Electrochemical Deposition of Co-Species on Carbon Fiber Paper:
[0074]The carbon paper, 1 cm×2.5 cm) was first soaked with ethanol, and then oxidized in 0.5 M H2SO4 solution with cyclic voltammetry for 10 cycles between 1.5 to 2.3 V (vs. Ag / AgCl, in saturation KCl solution). The oxidized carbon paper (1 cm×1 cm) was then immersed into a 0.1 M Co(NO3)2 solution for the electrodeposition of Co-precursor. A Pt foil and an Ag / AgCl (in saturation KCl solution) electrode were used as the counter and reference electrodes respectively. Electrodeposition was performed at a constant current mode (−10 mA / cm2) from 10 to 60 min in a PGSTAT 302N Autolab workstation. The as...
example 2
Preparation of a Co3O4 / Carbon Paper OER Electrocatalyst
[0079]Co3O4 / carbon paper was prepared under the same experimental conditions used in Example 1 with the exception that carbon-containing layer was omitted. The Co3O4 catalyst loading amount on carbon paper (50 min) was determined to be 12.6 mg using a high precision weighing balance.
example 3
Preparation of OER Electrocatalyst Comparative Samples
CoO / Carbon Paper (Example 3A)
[0080]The cobalt precursor was deposited on carbon paper using the procedure in Example 1 and then heated under vacuum treatment for 1 hour to yield CoO / carbon paper.
Co3O4 / Carbon Paper (Example 3B)
[0081]The cobalt precursor was deposited on carbon paper using the procedure in Example 1, and then heated in air at 350° C. for 5 h to yield Co3O4 / carbon paper. The Co3O4 catalyst loading amount on carbon paper for Examples 3A and 3B was determined to be 12.6 mg±2 mg using a high precision weighing balance.
Preparation of Nafion Coated Co3O4 / Carbon Paper (Example 3C) and RuO2 / Carbon Paper (Example 3D)
[0082]The Co3O4 and RuO2 powder were prepared by directly annealing Co(NO3)2.6H2O and RuCl3 precursors in a porcelain boat and placed in a muffle furnace, and then heated to 350° C. with a ramp of 2.5° C. / min and maintained for 5 h in air. After that, the furnace was allowed to cool to room temperature. Nafion (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com