Compositions and methods for treating beta-hemoglobinopathies
a beta-hemoglobinopathies and beta-hemoglobin technology, applied in the field of molecular biology, can solve the problems of limiting the treatment of patients with allogenic bone marrow transplantation, affecting the survival rate of patients, so as to achieve low overall toxicity, high engraftment, and efficient and high engraftment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
cal Testing of the TL20c-rGbGM-7SK / Sh734 Vector
[0309]Overview
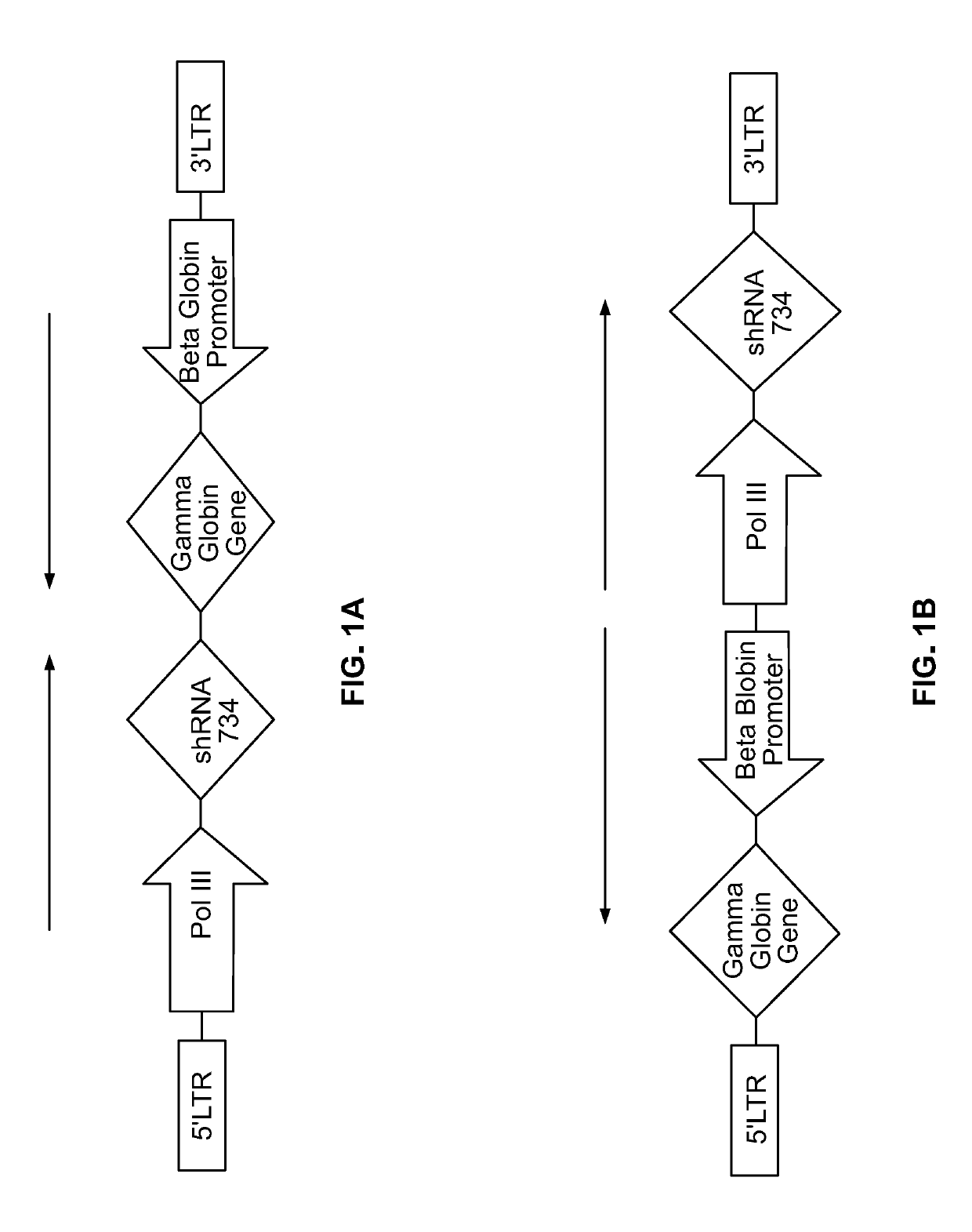
[0310]The pTL20c-rGbGM-7SK / sh734 dual therapeutic lentiviral vector construct was identified using a functional screen in K562 cells that compared the effect of position and orientation of the transgenes relative to each other on transgene expression and in vitro 6-TG selection. pTL20c-rGbGM-7SK / sh734 transduced K562 cells selected in 6TG culture demonstrated long term stability and expression of the γA-globin transgene normalized to VCN equivalent to cells transduced with parental GbGM lentiviral vector or CAL-H that were not treated with 6TG. These findings indicated that functional expression of the sh734 and the corrective sGbGM gene driven by different promoters was mutually exclusive and that regulation of sGbGM was lineage dependent. Using an in vitro model of human erythroid differentiation, we showed that CD34+ HSCs transduced with the CAL-H lentiviral vector constitutively expressed sh734 in extended cultures at ...
example 3
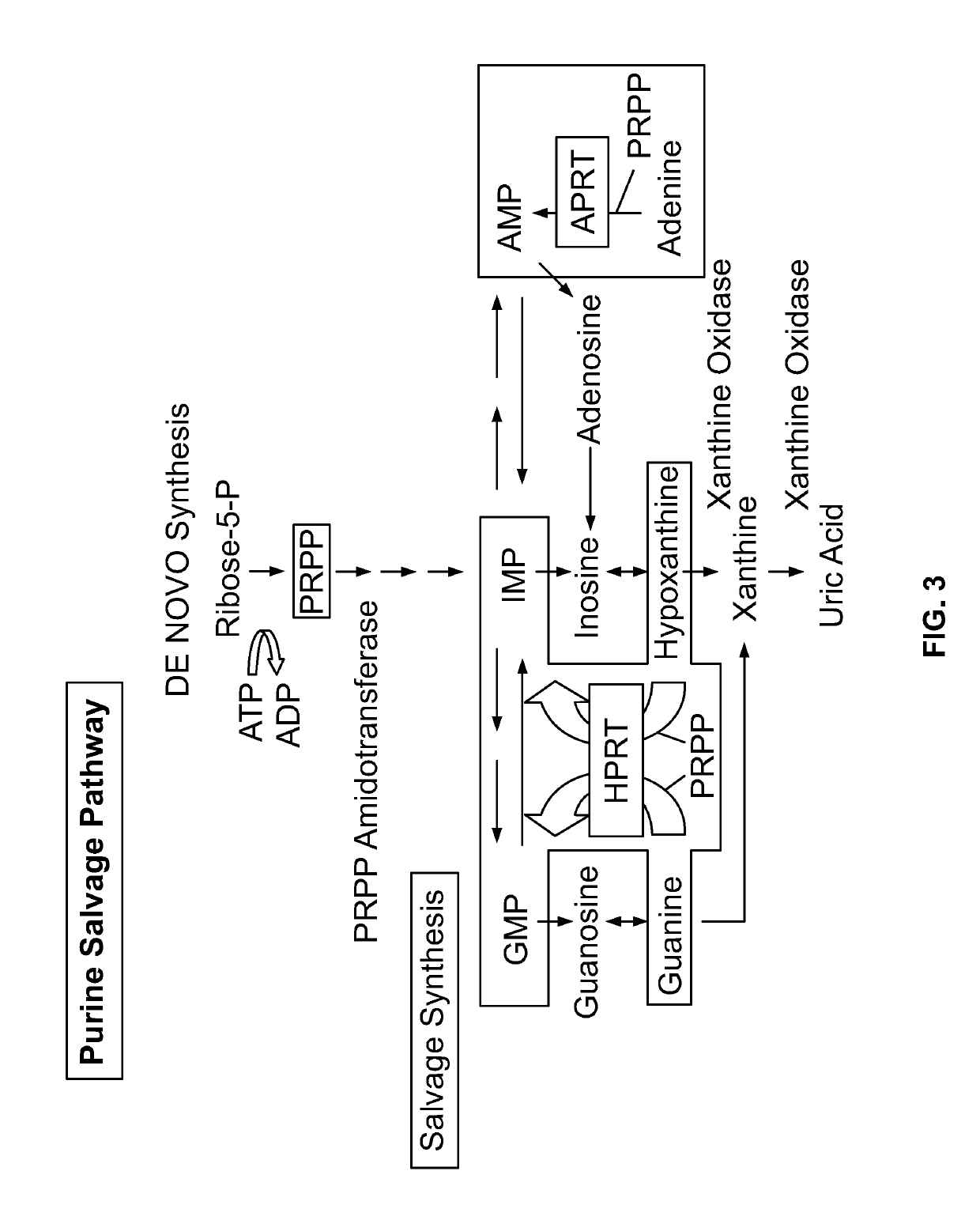
Polymerase II (Pol-II)-Dependent shRNA for Knock Down of HPRT and its Applications for 6-TG Selection
[0357]It has been well known that some polymerase III-dependent short-hairpin RNAs have overexpression issues and can induce acute cytotoxicity. Some pol III promoters, e.g. the U6, may lead to a much higher expression of short-hairpin RNAs (see Mol Ther. 2006 October; 14(4):494-504, which suggests the use of a pol II promoter driven shRNA to solve any toxicity issue), the disclosure of which is hereby incorporated by reference herein in its entirety). This is an important concern when considering the use of RNA interference (RNAi) as a potential therapeutic approach, especially in stem cell gene therapy. Here, polymerase II was used as alternative promoter to express microRNA so as to effectuate knockdown of the expression of HPRT. A CRISPR / Cas9 gene editing approach was utilized, and a Cas9 with a single guide RNA (Cas9 RNP) targeting CCR5, together with a single-stranded DNA oligo...
example 4
ing Prior to Hematopoietic Stem Cell Transplantation
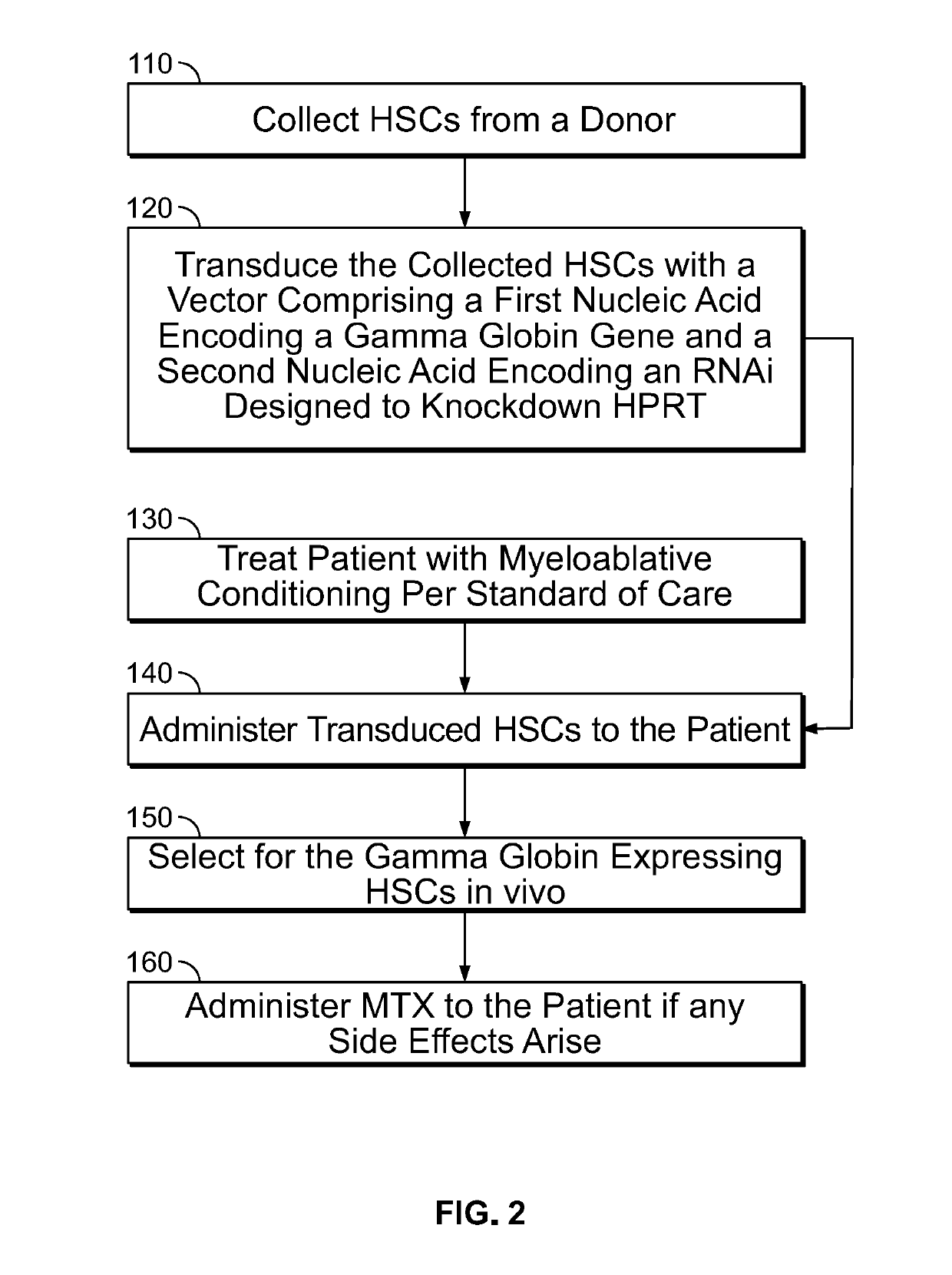
[0361]Hematopoietic stem cell transplantation (HSCT) is widely used to treat hematological malignancies and also offers curative therapy for patients with hemoglobinopathies, congenital immunodeficiencies, and other conditions, including infectious diseases such as HIV / AIDS. However, the ability of HSCT to cure this broad range of non-malignant diseases is severely underutilized. The obstacles to using allogeneic HSCT in these diverse conditions relate primarily to the frequency of life-threatening graft-versus-host disease (GVHD), of acute complications that result from the cytotoxic effects of conditioning, such as mucositis and infections, and of long-term, irreversible complications that arise from the genotoxic effects of conditioning, such as infertility. Autologous HSCT using genetically corrected cells would avoid the risk of GVHD, but the genotoxicity of conditioning remains a substantial barrier to the development of this...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com