Imidazopyridine and Oxazolopyridine Derivatives and Analogs Thereof, Methods of Preparation Thereof, Methods of HIF-2A Pathway Inhibition, and Induction of Ferroptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0327]Synthesis Schemes, Methods and Procedures:
Dimethyl (5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl)carbonimidodithioate (S1)
[0328]To a flame-dried, 100 mL round bottom flask equipped with a magnetic stir bar was added 5-(4-fluorophenyl)-1,3,4-oxadiazol-2-amine (1.00 g, 5.58 mmol) and DMF (10 mL). The reaction was cooled to 0° C., and aqueous NaOH (20 M, 0.31 mL) was added dropwise. Upon complete addition, the reaction was allowed to stir for 10 minutes followed by dropwise addition of CS2 (0.62 mL). The resulting solution was allowed to warm to room temperature over a period of 30 minutes. The flask was again cooled to 0° C., and iodomethane (1.6 g) was added dropwise. Upon addition, a yellow precipitate formed. The reaction was allowed to proceed for 30 minutes, and completion was confirmed by LC-MS. The reaction contents were then poured an Erlenmeyer flask with 50 mL of H2O, and the resulting precipitate was collected by vacuum filtration. The yellow precipitate was recrystallized...
example 2
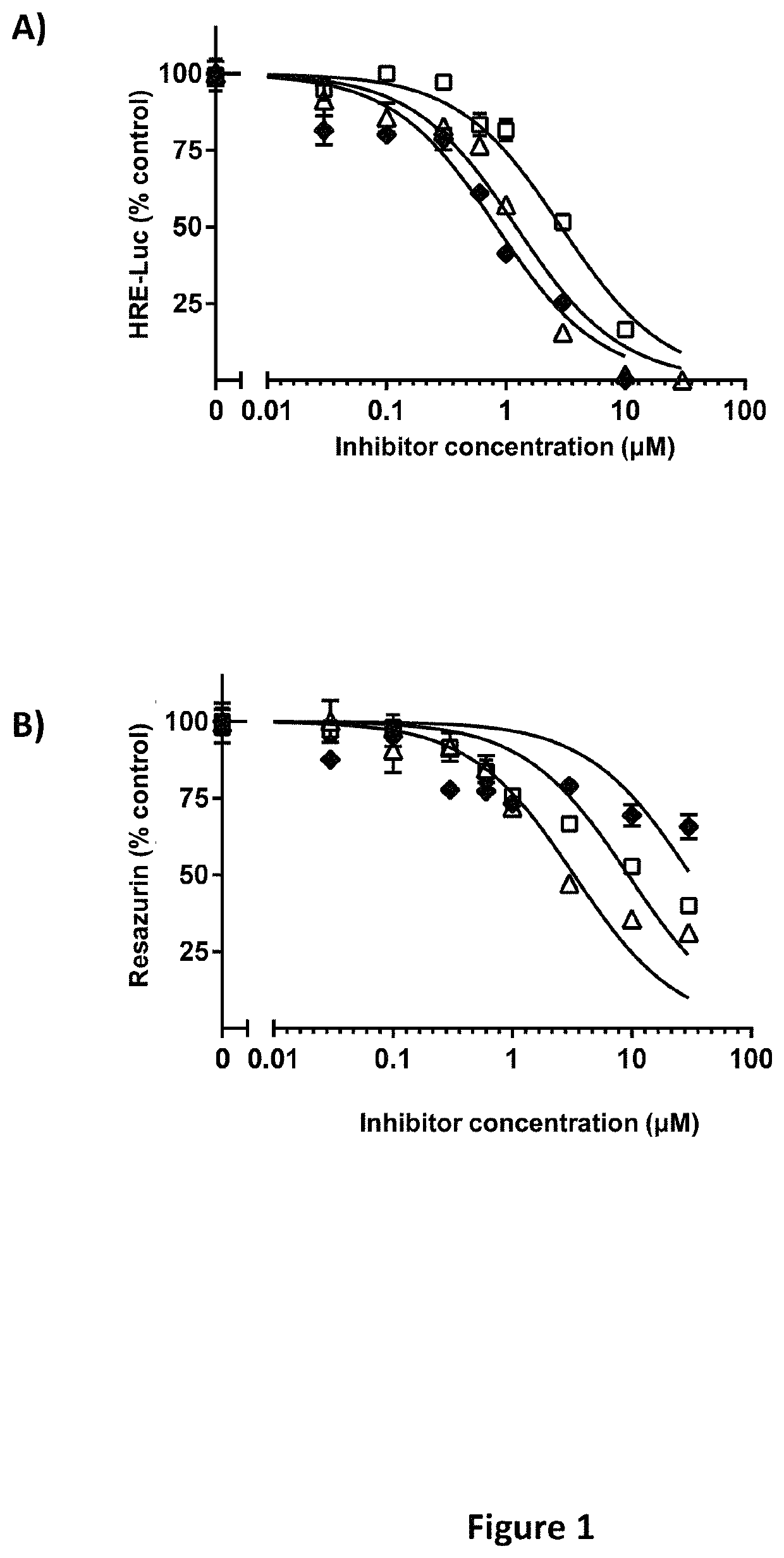
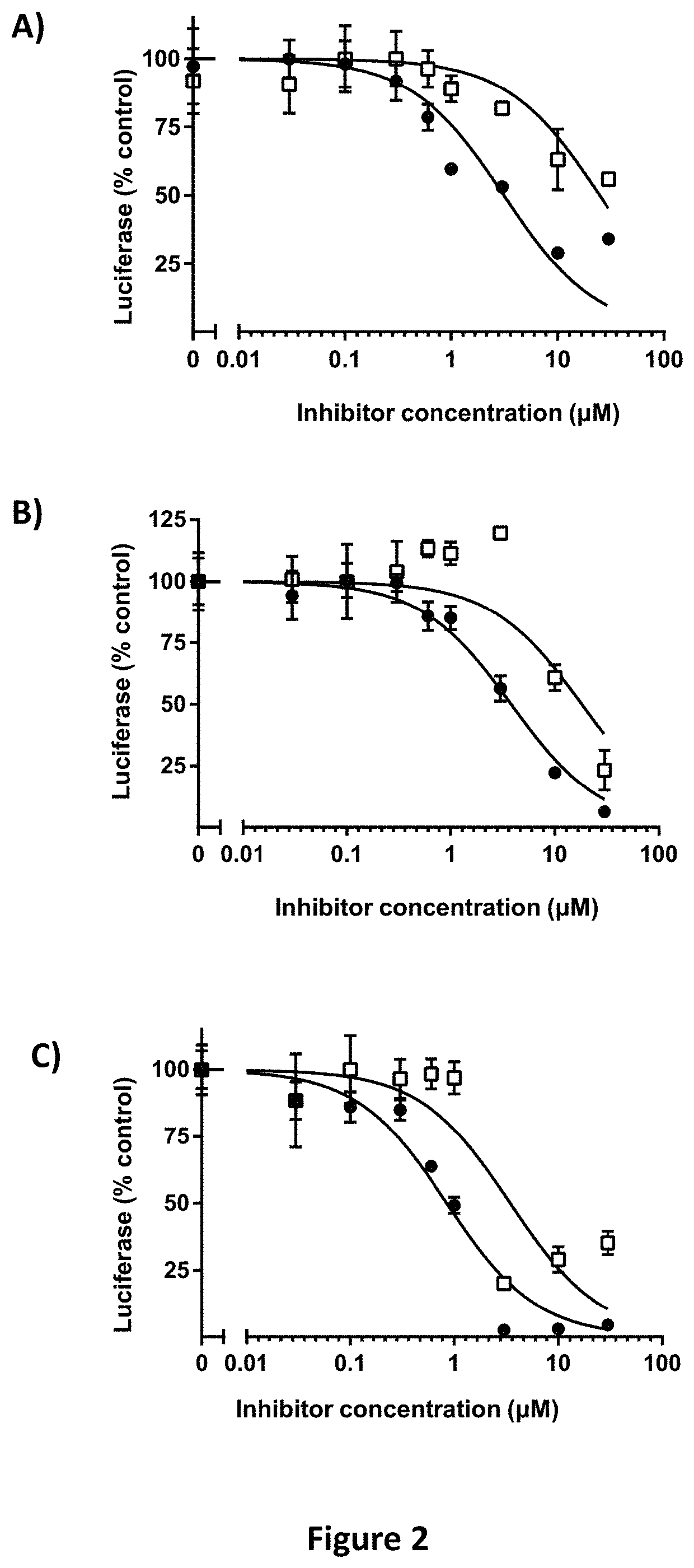
[0399]Hypoxia-Responsive Element-Driven Luciferase Screens to Identify Inhibitors of HIF-2α Transcriptional Activity.
[0400]Screens for inhibitory activity of compounds described herein were performed using 786-0 CCRCC cells that stably express HRE-Luc: 5 copies of the hypoxia-responsive element (HRE) fused to the pGL3 luciferase reporter (Promega Corp, Madison Wis.). 786-0 cells are pVHL deficient and thus constitutively express HIF-2α independently of cellular oxygen tension (Maxwell, Wiesener et al. 1999). Since 786-0 cells lack HIF-1α, HRE-driven luciferase activity is primarily HIF-2α driven, and has been previously validated (Koh, Lemos et al. 2011). Cells were maintained at log phase growth in Dulbecco's minimal essential media (DMEM) with 10% FBS in a humidified incubator at 37° C. with 5% CO2. For screening assays, cells were seeded at a density of 4,000 cells in 50 μl of complete media / well in quadruplicate wells / point in a 96-well plate. 24 hours later, 50 μl of a 2× conce...
example 3
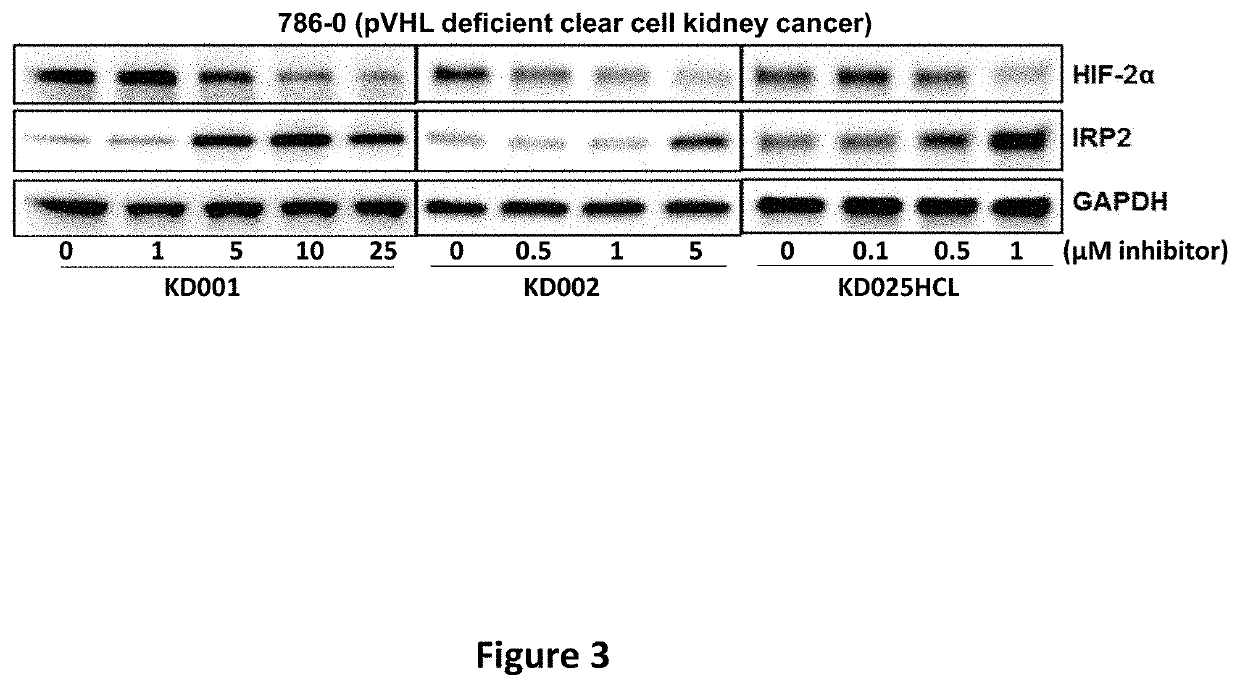
[0403]Western Blots to Determine Impact of Compounds on HIF-2α and Cellular Iron-Sensing Machinery.
[0404]Western blots were performed to determine the effects of the compounds on levels of HIF-2α and other relevant proteins. 786-0 (pVHL deficient clear cell kidney cancer cells) were purchased from ATCC were plated at 1×10E5 cells / well in 2 ml DMEM with 10% FBS / well in 6-well tissue culture plates. Cells were allowed to adhere overnight in a humidified incubator at 37° C. with 5% CO2, after which the appropriate concentrations of compounds in DMSO were added. DMSO concentrations were kept constant in all wells. After 24 hour's exposure to the compounds, cells were lysed and subjected to western blotting according to standard protocols (Polek, Talpaz et al. 2003). Antibodies for HIF-2α and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Danvers, Mass.), whereas antibodies to iron responsive element binding protein 2 (IRP2) and Glutathion...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com