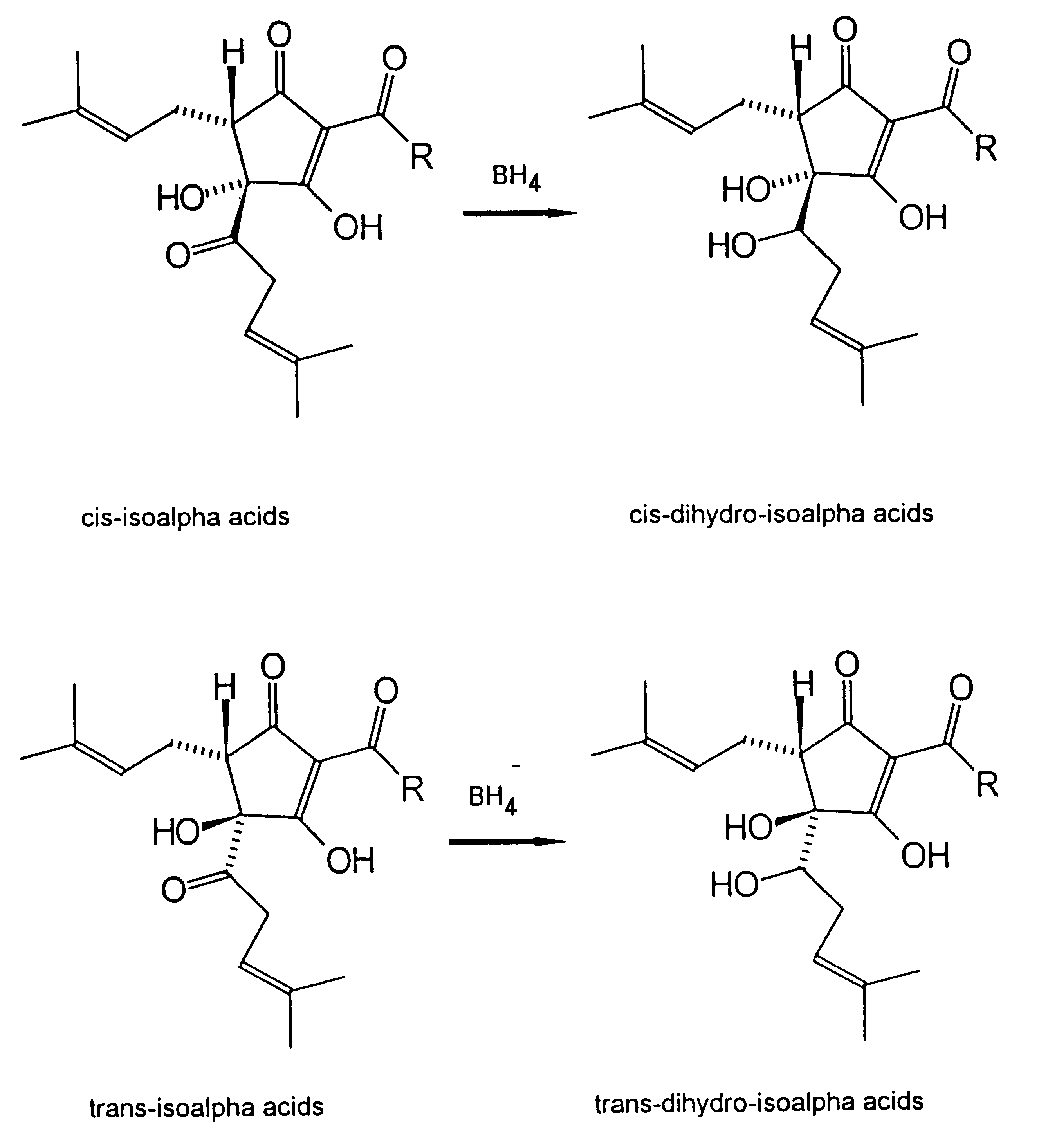
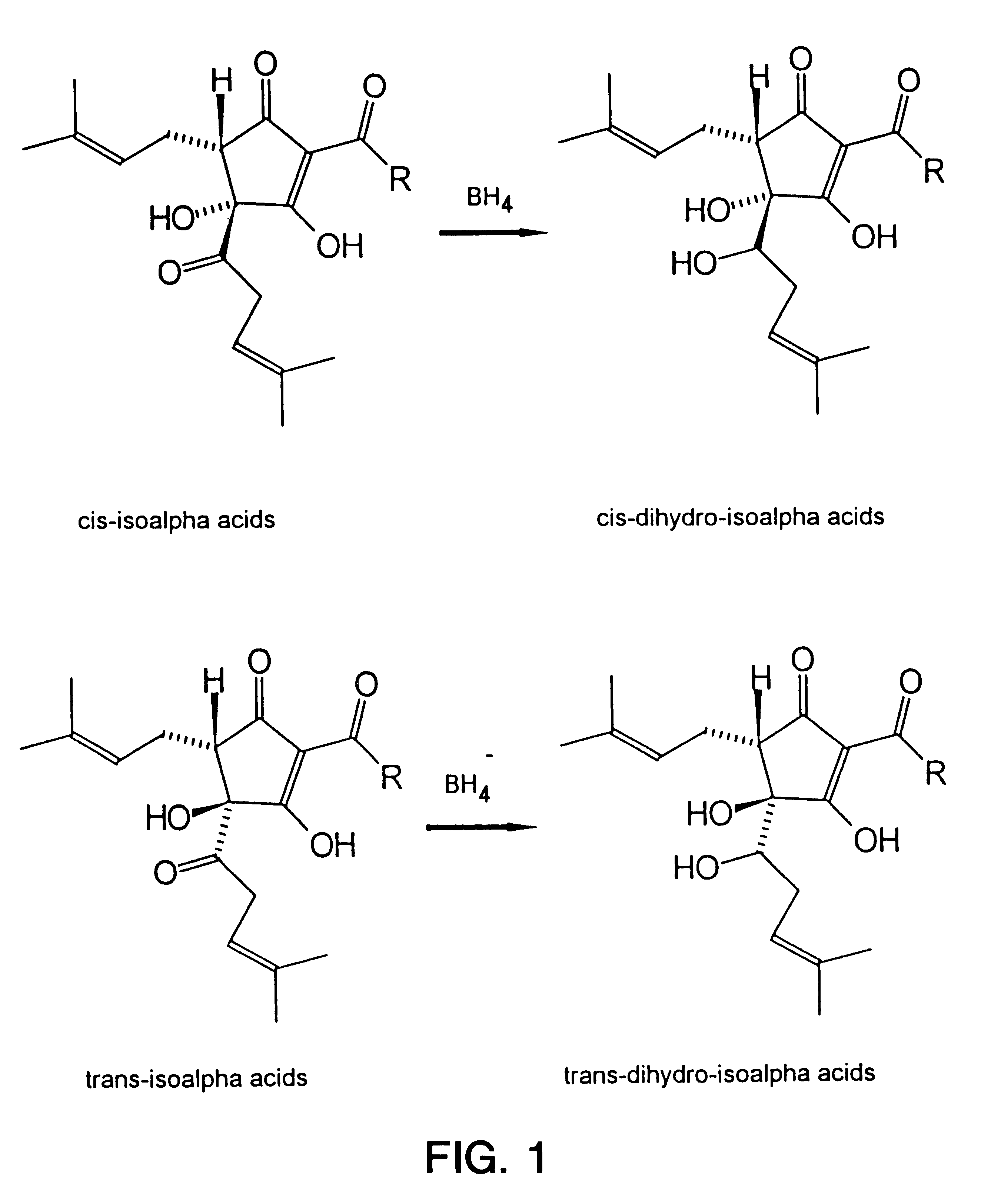
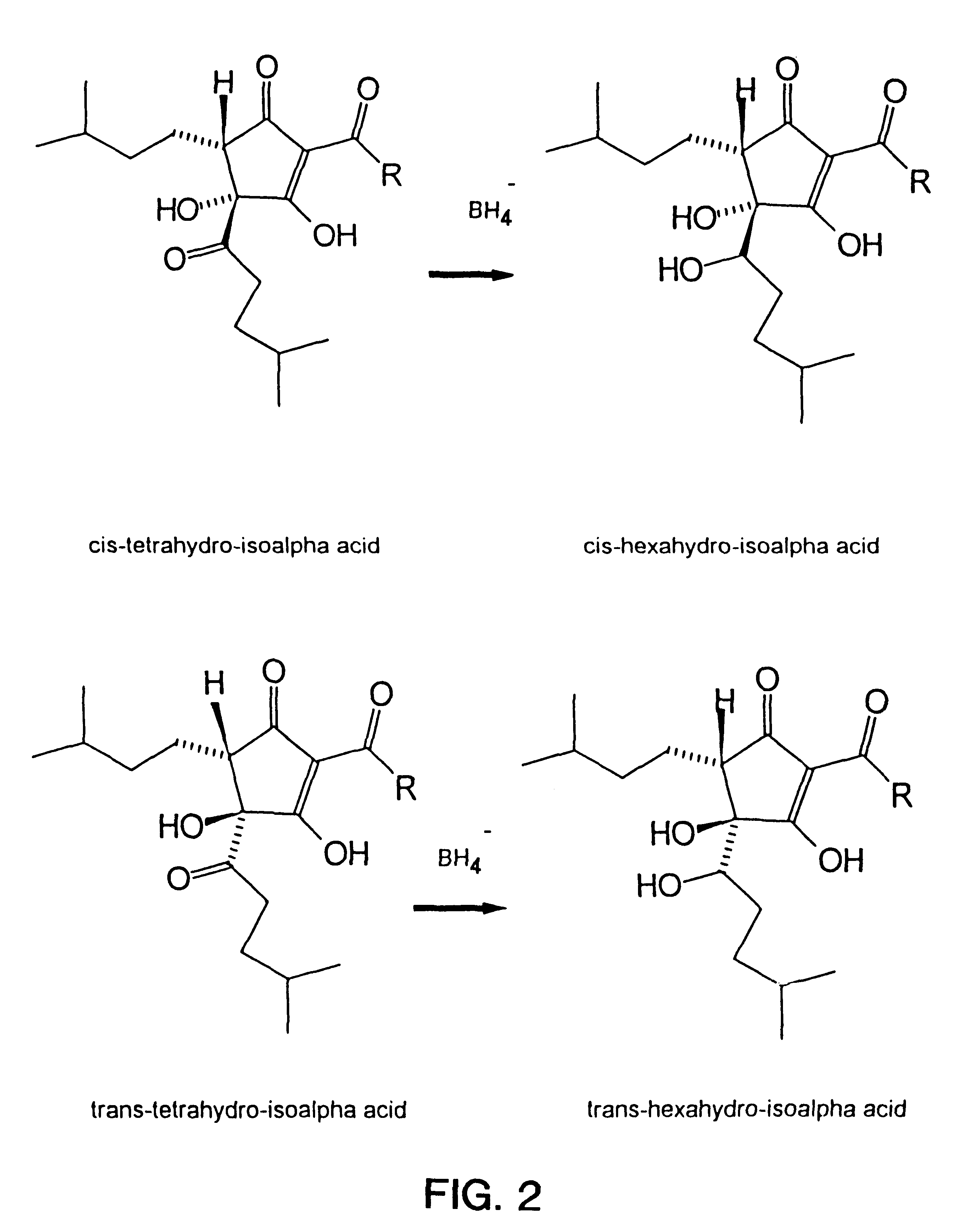
Dihydro and hexahydro isoalpha acids having a high ratio of trans to cis isomers, production thereof, and products containing the same
a technology of hexahydro isoalpha acids and which is applied in the field of dihydro and hexahydro isoalpha acids having a high production thereof, and products containing the same, can solve the problem that no prior art products have these qualities, and achieve high trans isomer ratio, stable phs, and increased critical ratio of trans to cis isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 thru 4
show variations on the preferred process for making a high trans , highly soluble DHIA and HHIA which, in turn, do not form hazes upon dilution to 1% in distilled water.
It will be noted that none of these products form insoluble precipitates on standing, and that they may be added directly to soft water to whatever concentration the brewer desires. It will also be noted that they do not form precipitates visible to the unaided eye or measurable haze upon direct injection into bottled beer. None of the prior art products have these qualities. Goldstein's NILUPs-free type DHIA all cis products and the all cis HHIA products presently available do not form clear solutions.
The purity of the hop acids must be exceptionally high if solutions of the high trans product are to remain clear. Substances eluting after the hop acid in the HPLC procedure must be less than 6%, preferably less than 4%, and most preferably less than 1% to 2%. Likewise, "waxes" which do not absorb uv light and which a...
example 1
Preparation of a Liquid DHIA by Reduction Without the Use of Solvent in the Reducing Medium.
52 g of a 29% aqueous solution of IA was added to 100 ml of water and the pH adjusted to 10.5 to improve solubility. 0.87 g of NaBH.sub.4 ( 0.55 molar equivalents ) in 75 ml of water were added, the solution heated to about 50.degree. C., and the reaction terminated after two hours. 50 ml of hexane, a C-6 hydrocarbon, was added, and the mixture cooled. The aqueous and hydrocarbon phases were agitated with phosphoric acid at a pH of 2.8, the aqueous phase containing boron discarded, and the organic phase was extracted with water to remove residual boron. The organic phase, containing the DHIA, was then partitioned against 100 ml of warmed distilled water brought to a pH of 8.4 with potassium hydroxide. (A pH of between about 7.6 to 8.8 is optimal for this separation, although higher and lower pHs may be used. Furthermore, concentrations of 20%, preferably 15%, and most preferably about 10% or ...
example 2
Preparation of DHIA from IA with the Use of Solvent.
500 g of a 25% (measured by total absorbance at 254 nm) aqueous solution of crude IA was added to 250 ml of a 4% solution of NaBH.sub.4 (0.75 molar equivalents). The pH was 11.4. 125ml of limonene, a C-10 hydrocarbon, was added, and the mixture heated with agitation for 3 hours at 70-75.degree. C. The mixture was cooled, and the organic phase separated from the aqueous DHIA phase. The organic phase was discarded. 200 ml of a light petroleum distillate (boiling point less than 100.degree. C.) was added to the aqueous phase, and the solution acidified to pH 2 with phosphoric acid. The aqueous phase was separated and discarded. The organic phase was washed once with water at a pH of about 3, and again at a pH of about 4 to 5 to eliminate all boron. The organic phase was then partitioned with 1000 ml of distilled water brought to a pH of 7.6 with potassium hydroxide and the phases separated. The aqueous DHIA phase was concentrated unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com