Liquid detergent composition
a technology of liquid detergent and composition, which is applied in the direction of detergent bleaching agents, detergent compounding agents, liquid soaps, etc., can solve the problems of weak bleaching power, difficult stable blending with the bleaching activator, and hardly suppressed hydrogen peroxide hydrolysis of the bleaching activator
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment a
[0029]The liquid detergent composition of the present invention contains (a) hydrogen peroxide or a compound forming hydrogen peroxide in water [referred to hereinafter as component (a)]; 0.1 to 10 mass % of (b) a bleaching activator [referred to hereinafter as component (b)], 45 to 80 mass % of (c) a nonionic surfactant [referred to hereinafter as component (c)], (d) water [referred to hereinafter as component (d)], (e) at least one or more compounds selected from boric acid, borax and borate [referred to hereinafter as component (e)], and (f) a polyol compound [referred to hereinafter as component (f)], said liquid detergent composition having a pH value of 4 to 7 at 20° C.
[Component (a)]
[0030]The liquid detergent composition of the present invention contains, as the component (a), hydrogen peroxide or a compound for forming hydrogen peroxide in water. The compound for forming hydrogen peroxide in water includes a percarbonic acid salt, a perboric acid salt and the like. The conte...
embodiment b
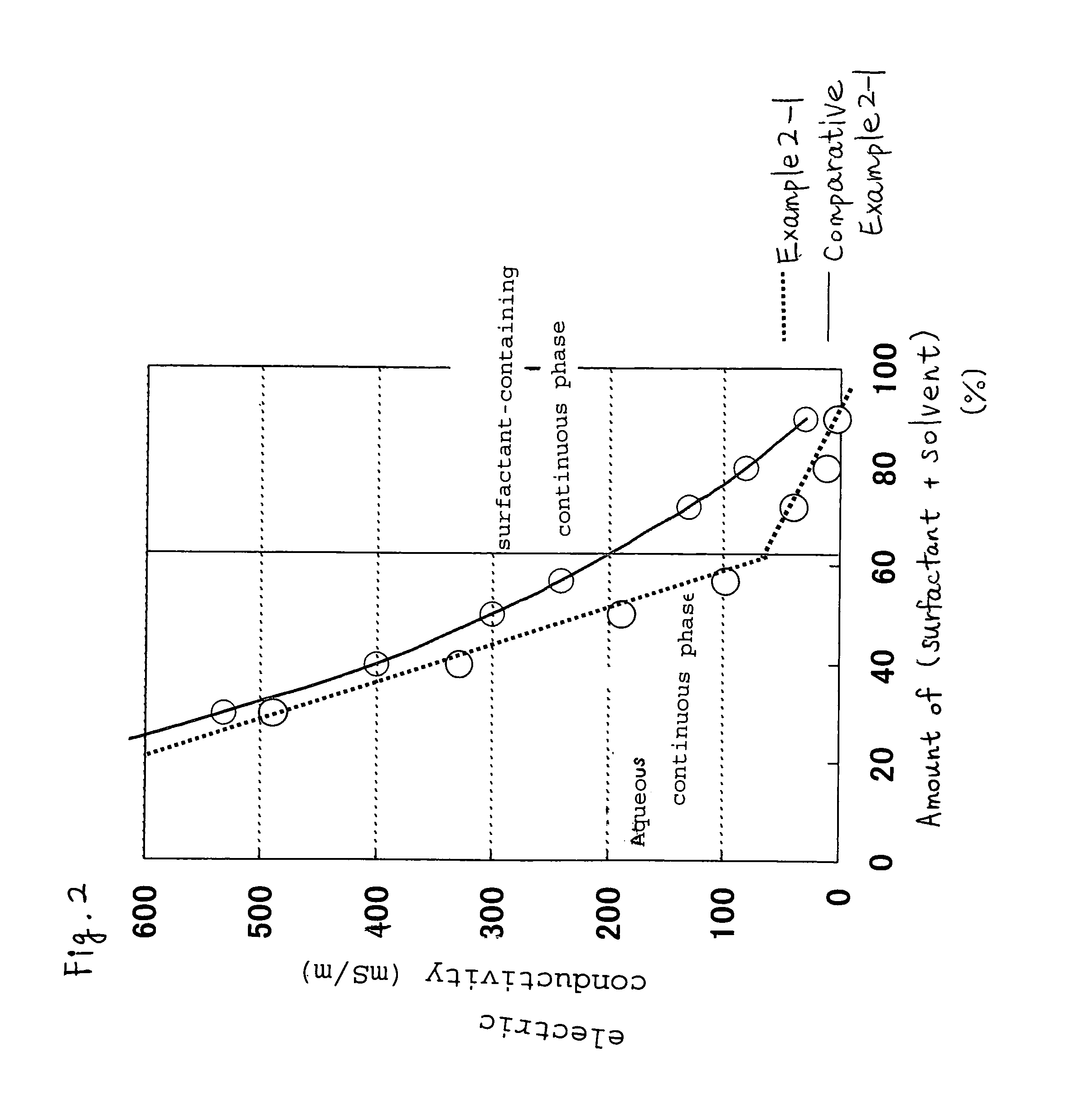
[0093]The liquid bleaching detergent composition of the present invention is a hydrogen peroxide-containing liquid bleaching detergent composition containing a water-in-oil emulsion (W / O emulsion) having aqueous liquid droplets dispersed in a surfactant-containing continuous phase.
[0094]The composition of the present invention contains hydrogen peroxide (referred to hereinafter as component (A)) as a bleaching base. From the viewpoint of solution stability, hydrogen peroxide in the form of a liquid is preferred to sodium percarbonate in the form of a powder.
[0095]The content of hydrogen peroxide (A) in the present invention is preferably 0.1 to 20 mass %, more preferably 0.5 to 10 mass %, even more preferably 1 to 6 mass %, based on the liquid bleaching detergent composition, and with these ranges given, an excellent bleaching effect can be obtained.
[Surfactant]
[0096]The composition of the present invention contains a surfactant (referred to hereinafter as compone...
examples
[0154]The present invention is described in more detail by reference to the Examples. The Examples are illustrative of the present invention and are not intended to limit the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass % | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com