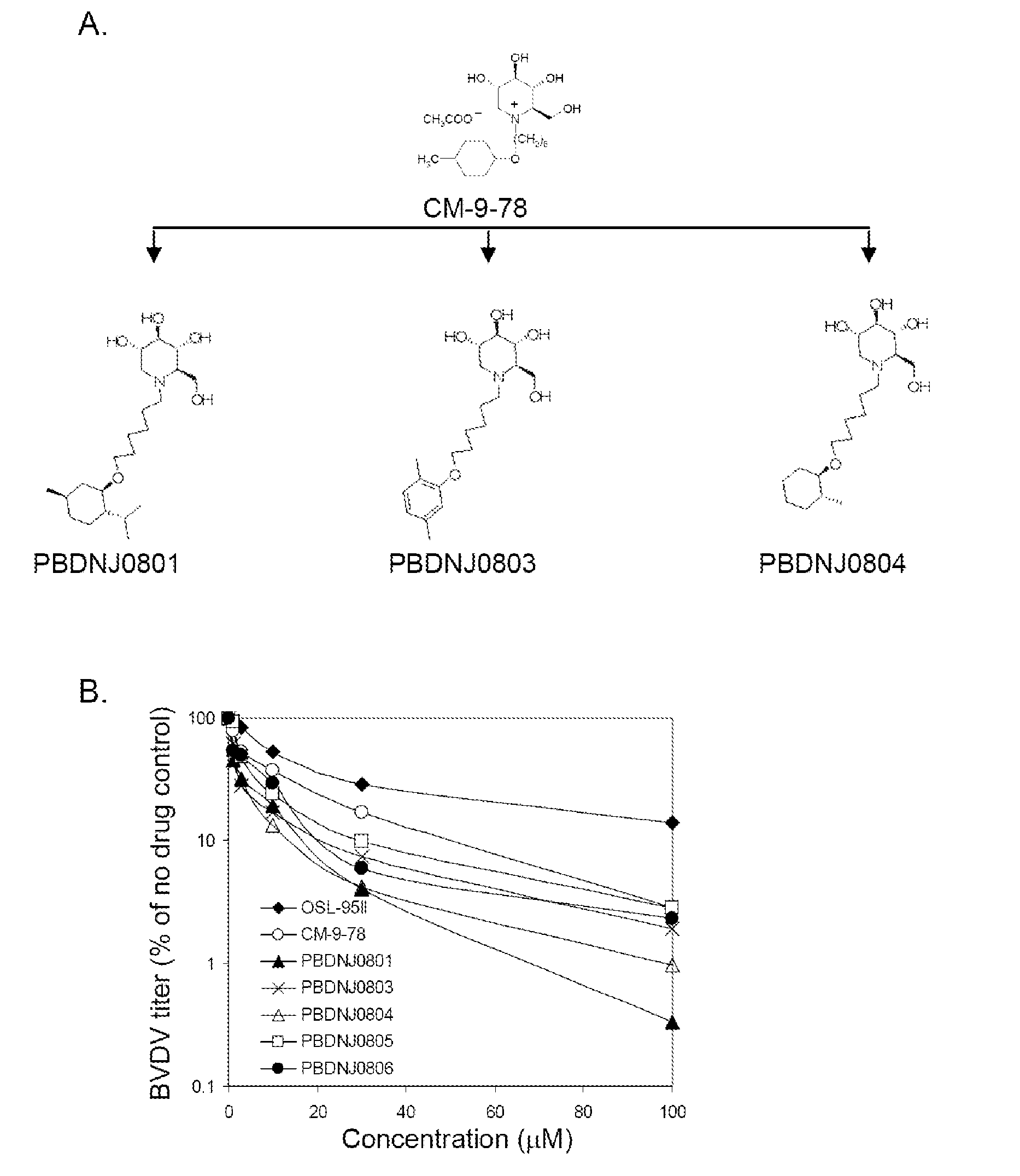
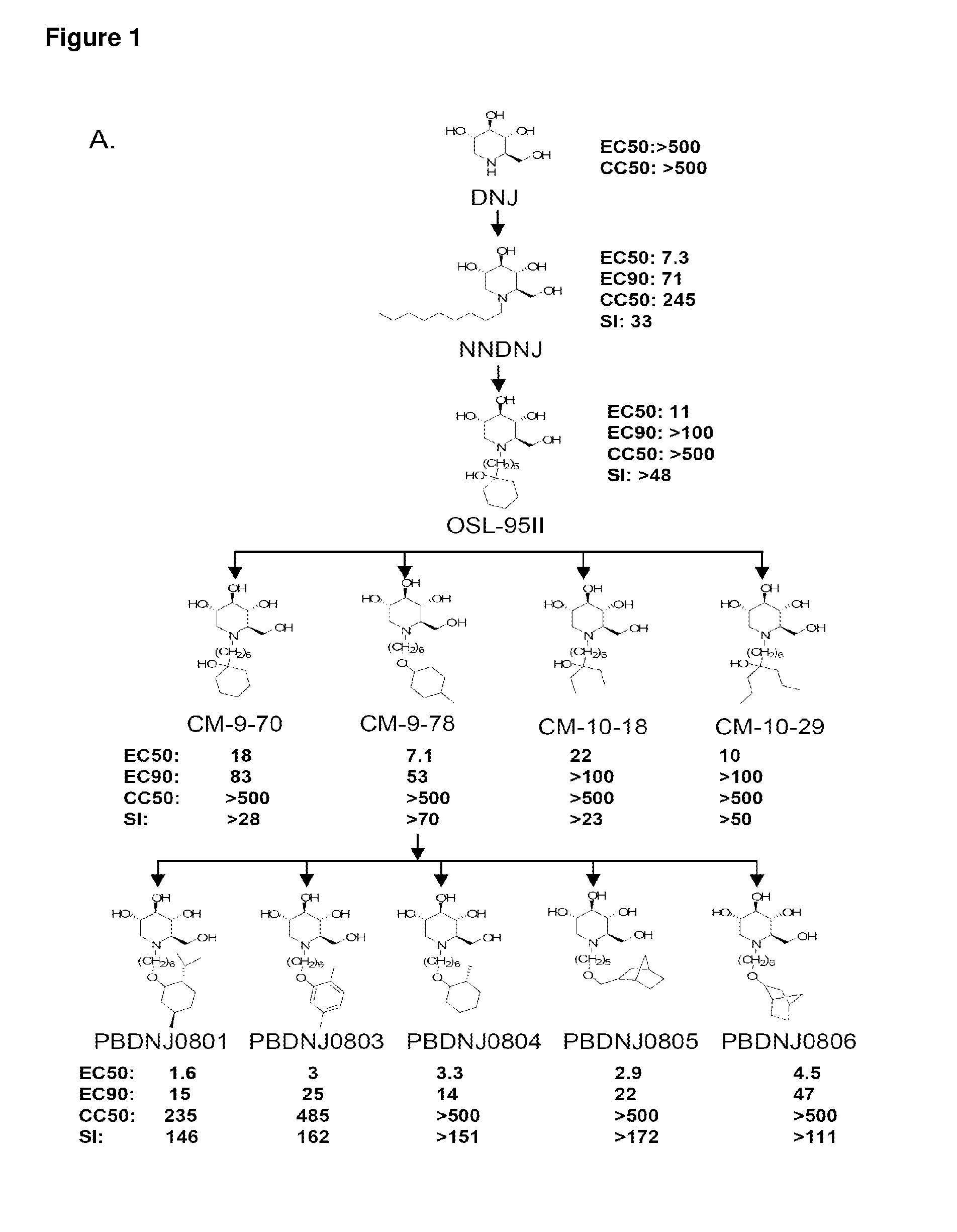
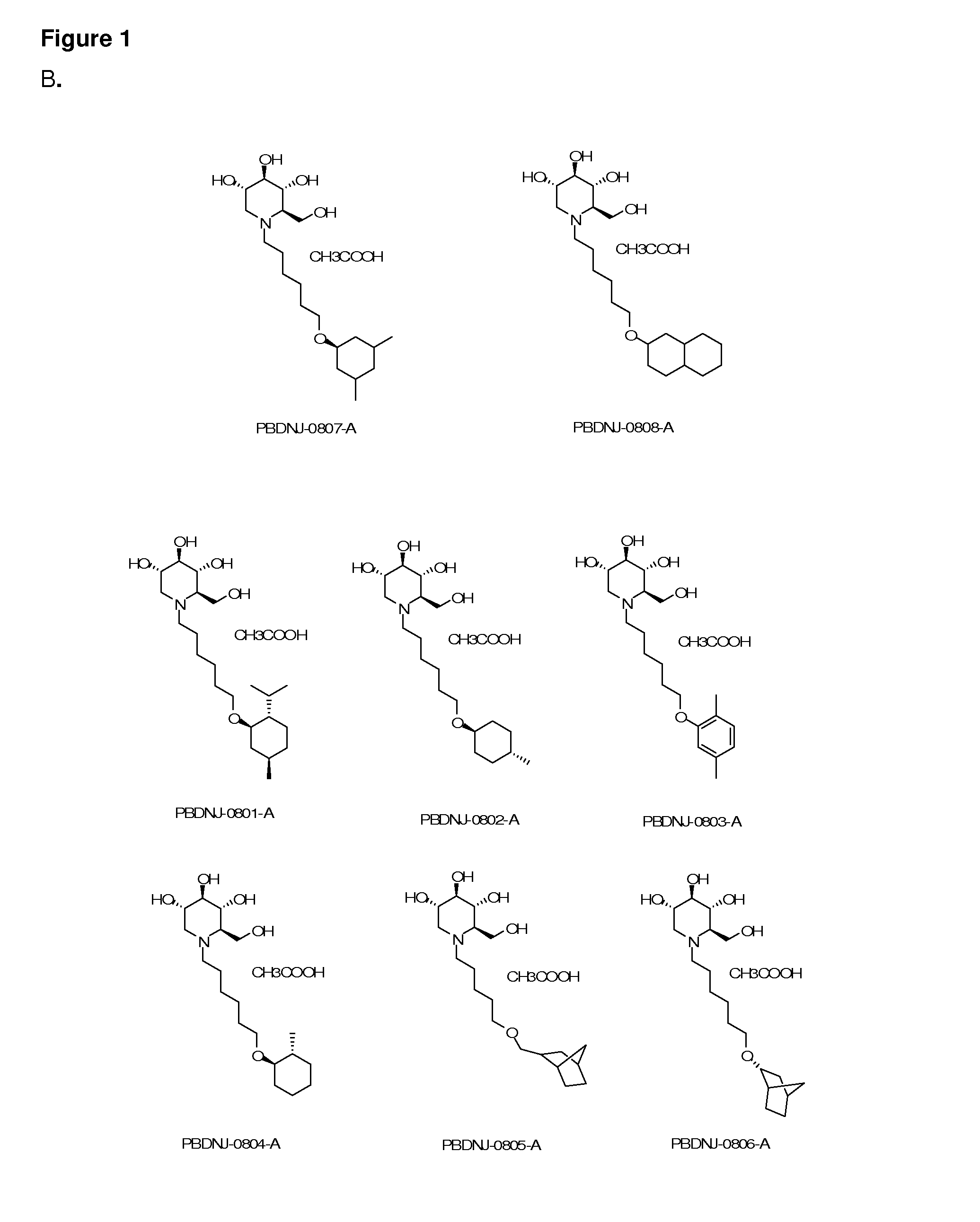
Imino sugar derivatives demonstrate potent antiviral activity and reduced toxicity
a technology of imino sugar and derivatives, which is applied in the field of antiviral compounds and compositions, can solve the problems of limited development of imino sugar glucosidase inhibitors, low efficacy and/or cytotoxicity, and misfolding and degradation of glycoproteins, and achieves low cytotoxicity, high inhibitory potency, and improved antiviral activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Introduction of Oxygenated and Terminal Ring Structures into the Nitrogen-Linked Alkylated Side Chain of Deoxynojirimycin
Materials and Methods
[0039]Cells and viruses. BVDV-free MDBK cells (CCL 22) were obtained from the American Type Culture Collection and propagated in DMEM / F12 essential medium supplemented with penicillin (500 U / ml), streptomycin (500 U / ml), and 10% heat inactivated horse serum (Invitrogen). Cells were maintained in a humidified incubator at 37° C. with 5% CO2. BVDV (NADL strain). For infections, virus inoculum was added in complete medium and adsorbed for 1 hour at 37° C., the inoculum was then removed, the cells washed once with medium and fresh medium containing compounds added. Virus stocks were prepared by freeze-thawing the infected cells and culture supernatant three times followed by centrifugation at 1,000 g for 5 min. Stock titers were determined, and stocks were aliquoted and stored at −80° C. WNV was obtained from a cDNA clone of a human 2002 isolate f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| specific structure | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com