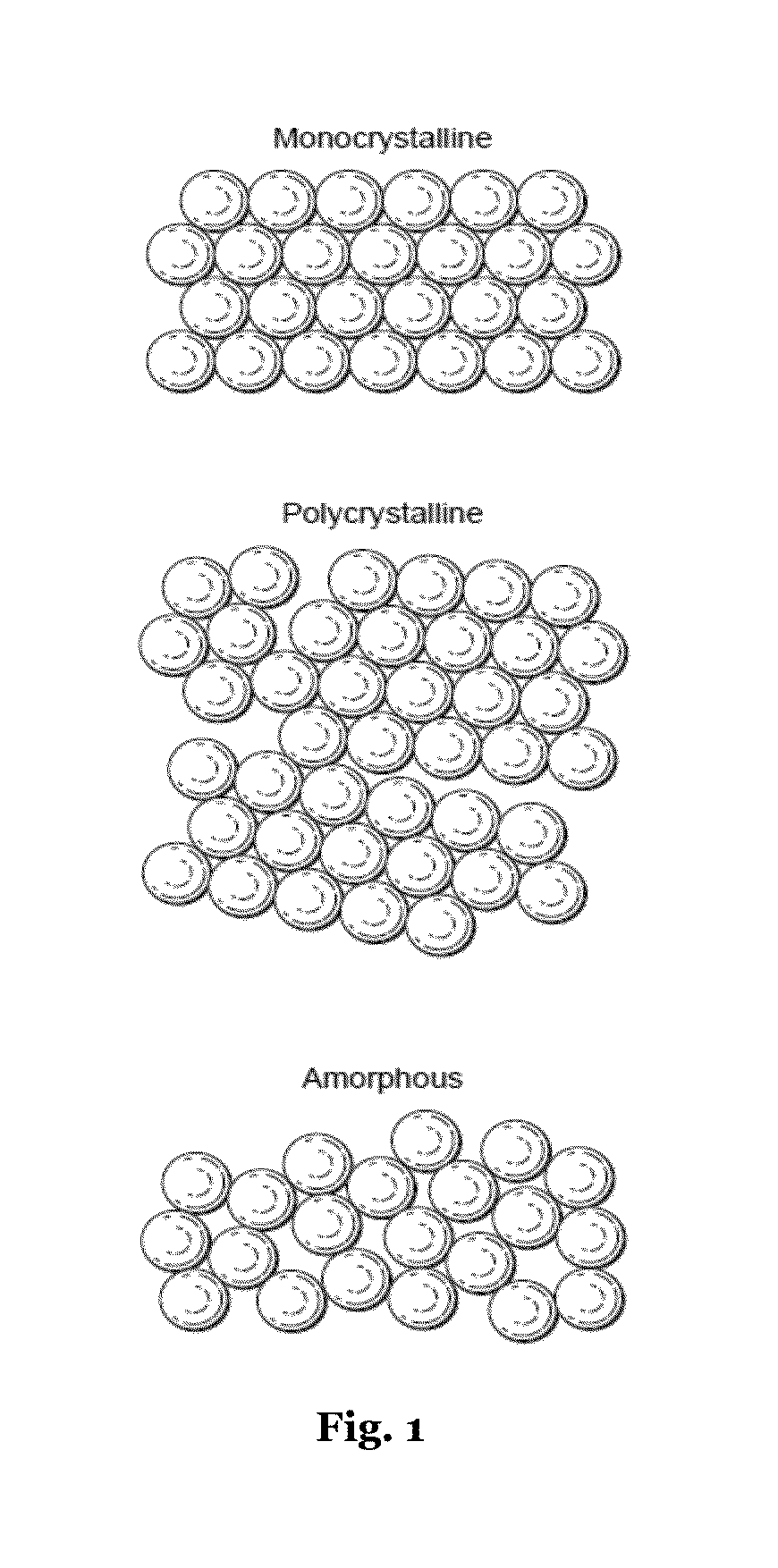
Aluminum metal organic framework materials
a technology of organic frameworks and metals, applied in the direction of group 3/13 element organic compounds, group 4/14 element organic compounds, etc., can solve the problems of low chemical stability, hampering the application of the industry, and few stable mofs, especially in single crystal form, and achieve excellent solubility, excellent starting materials, and high symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Al3O(ABTC)6-PCN 250 (Al)
[0230]
[0231]10 mg of [Al3O(OOCCH3)6.3CH3CN][AlCl4] and 10 mg of ABTC were dissolved in 2 ml of DMF, then 0.5 ml of acetic acid was added. The solution was sealed in a 4 ml vial and put into oven at 150° C. for 5 days. After cooling down to room temperature, light yellow crystals were harvested.
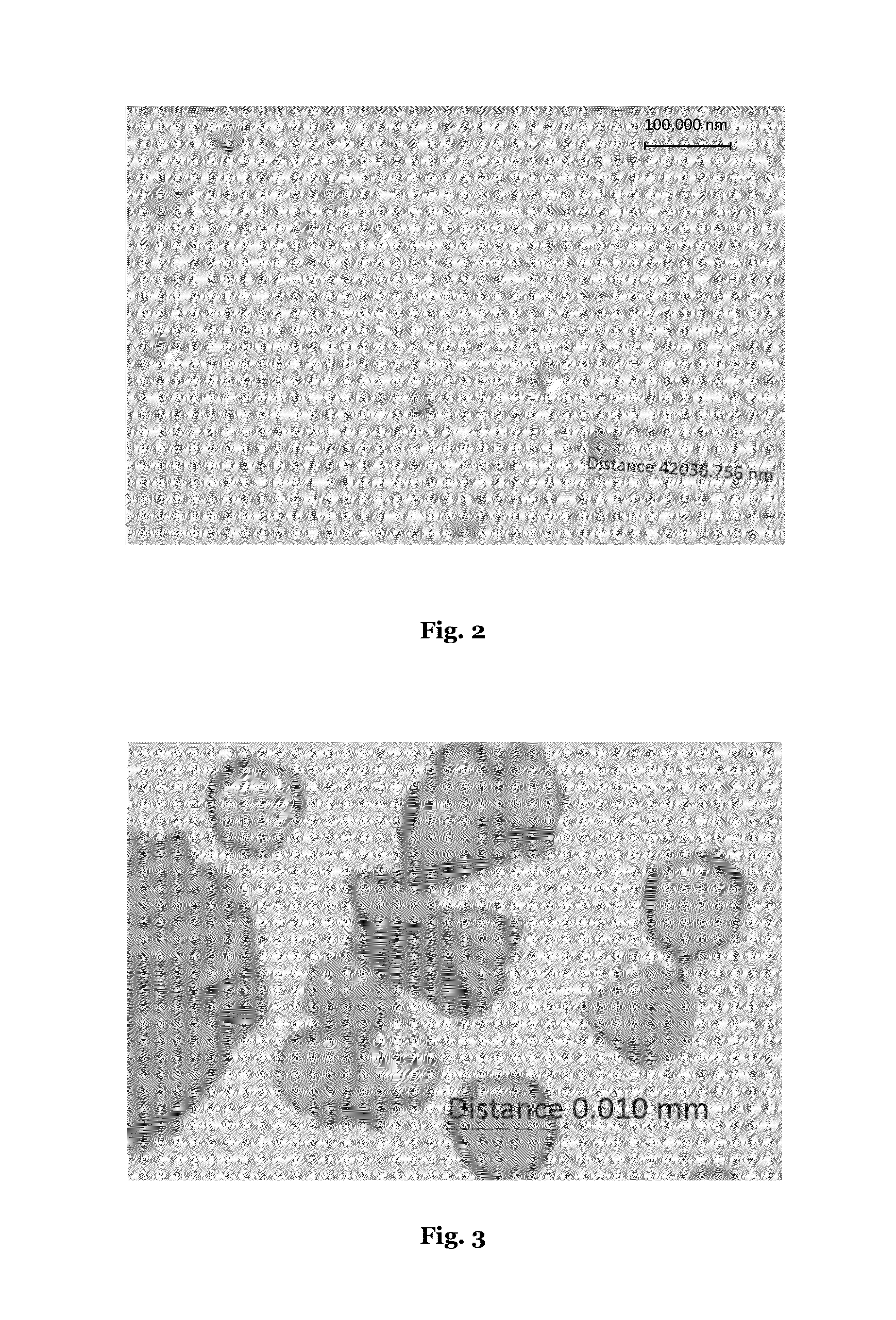
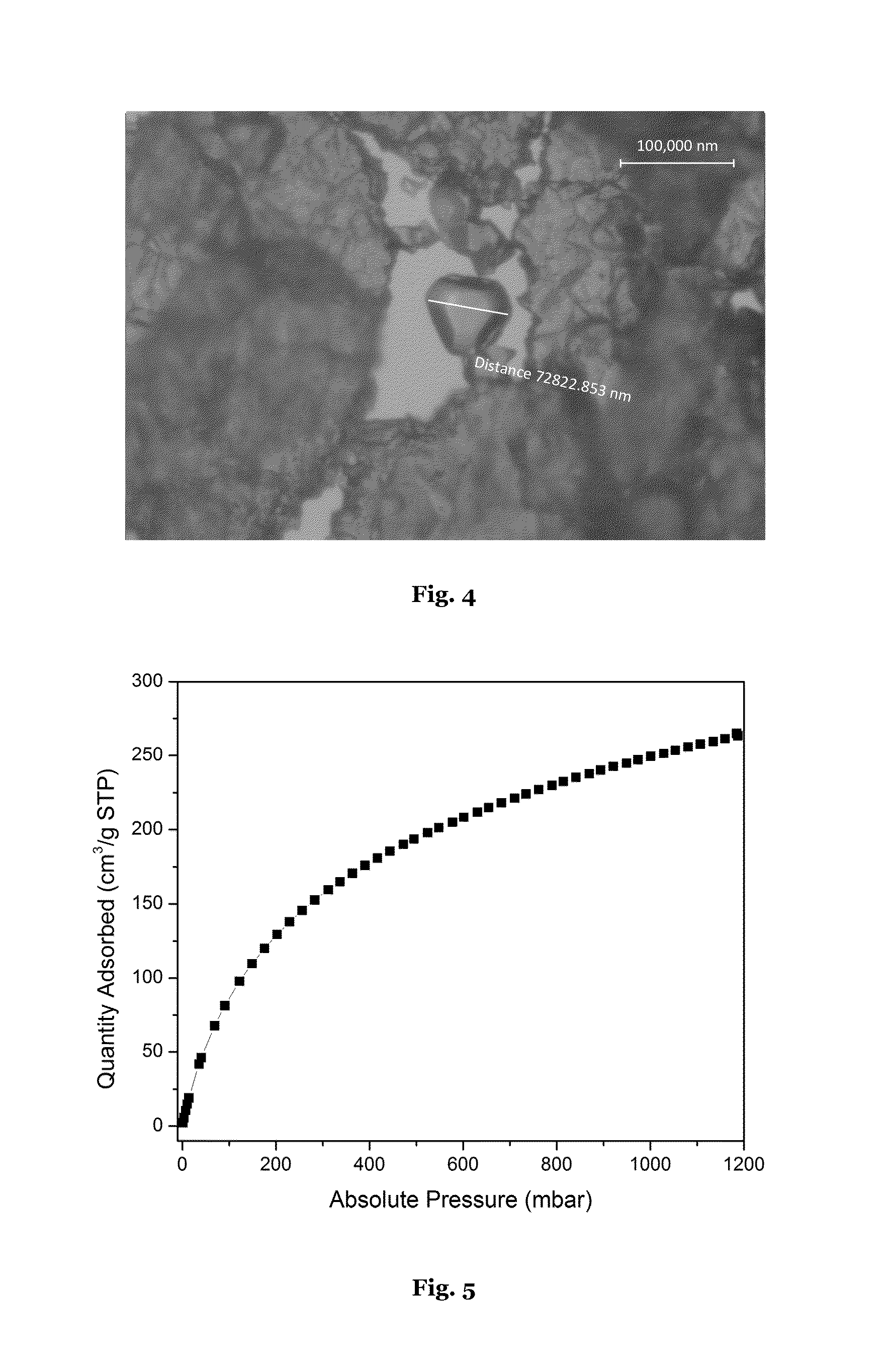
[0232]Optical microscope images of PCN-250 (Al) (Example 1) are shown in FIGS. 2-4. Crystal sizes of 42 μm, 10 μm, and 72 μm respectively were observed.
[0233]The crystal data and structure refinements for a single crystal of PCN-250 (Al) (Example 1) are shown in Table 1.
[0234]
TABLE 1PCN-250-AlFormulaC9 H6 Al O5.33Formula weight226.45Crystal Color / ShapeLight Yellow BlockCrystal SystemCubicSpace GroupP43na (Å)21.6035(10)V (Å3)10082.60(8)Z24dcalcd. (g / cm3)0.895μ(mm−1)0.121F(000)2776θmax [deg]26.37Completeness98.8%Collected reflections3427Unique reflections3238Parameters145Restraints3Rint0.0308R1[I > 2σ(I)]0.0386wR2 [I > 2σ(I)]0.1241R1 (all data)0.0408wR2 (all ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| BET specific surface area | aaaaa | aaaaa |
| BET specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com