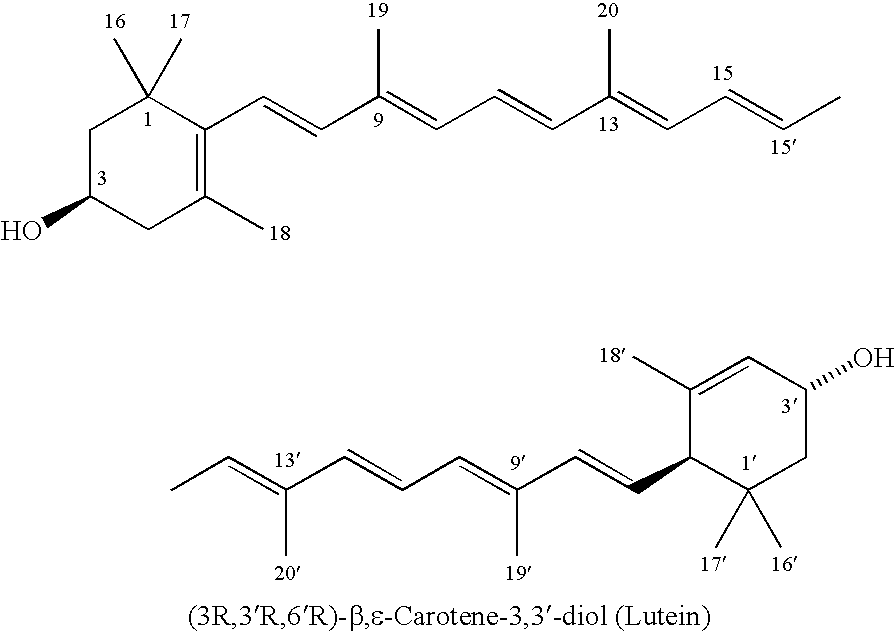
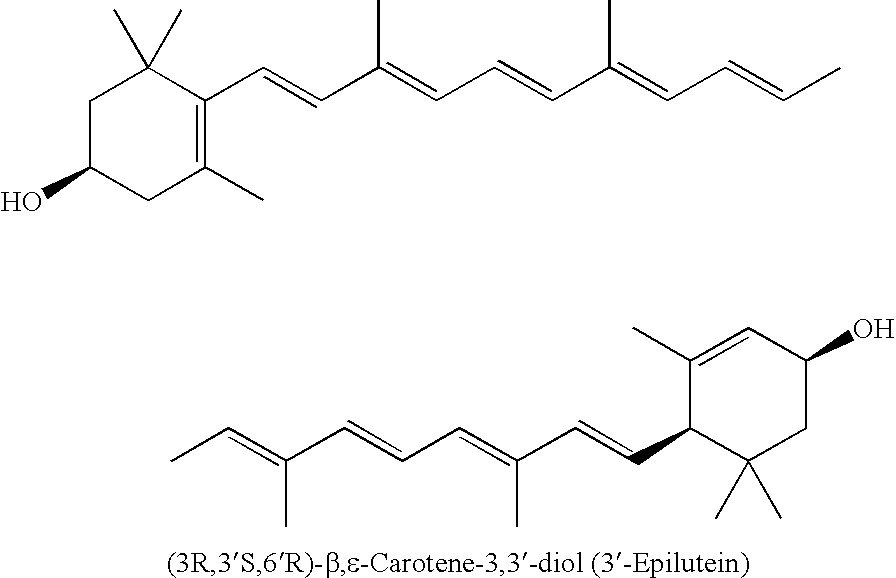
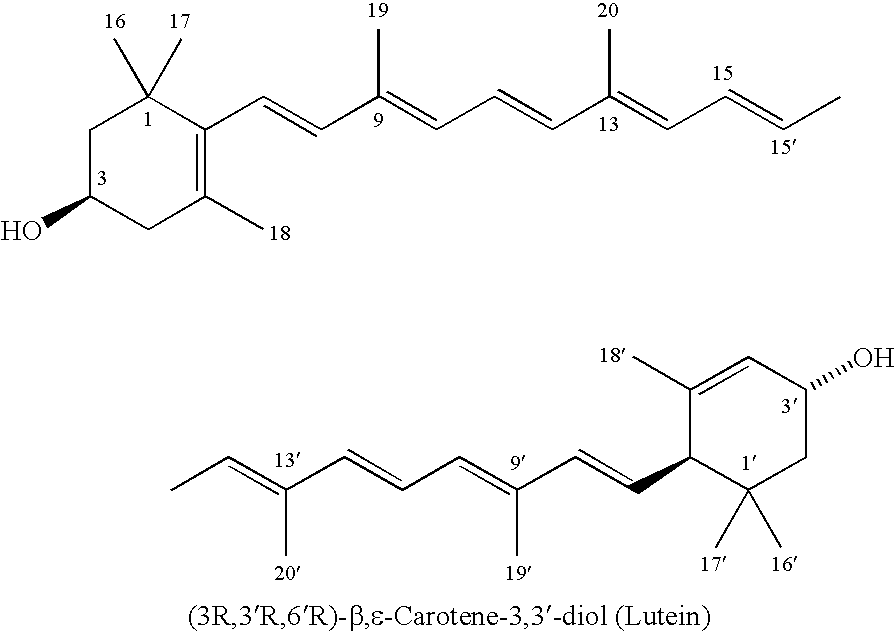
Process for isolation, purification, and recrystallization of lutein from saponified marigold oleoresin and uses thereof
a saponified marigold and purification technology, applied in the field of saponified marigold oleoresin purification, purification and recrystallization of lutein, can solve the problems of high risk of direct oxidation of carbon, uncommercially available pure lutein suitable for human use, uneconomical pure lutein in large quantities of green vegetables, etc., to achieve safe and effective color additive in human food
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0030]Saponified marigold oleoresin available commercially as “Kemin Yellow Oil” was obtained. It was previously processed in the following manner.
[0031]Upon receipt the flowers were tested for herbicides and pesticides in order to ensure that they meet qualifications for food ingredients. The completed oleoresin extract (200 g) was then subjected to saponification with aqueous potassium hydroxide. This was accomplished through continuous mixing under heat (65°-70° C.) of food grade aqueous potassium hydroxide (45%) and the oleoresin until greater than 98% of lutein was free from fatty acid esters. The saponification was normally completed within 35 minutes. The product was then homogenized with a mixture of distilled water (700 mL) / ethanol (300 mL, food grade):2.3 / 1 at room temperature for 30 minutes. The mixture was filtered off and the filtrate was discarded. The retained orange precipitate of lutein was washed with distilled water until the filtrate was almost colorless and the ...
example 2
[0033]Saponified marigold oleoresin (200 g) was homogenized with a mixture of distilled water / ethanol (food grade) at various ratios of 0° C.-10° C. for 30 minutes. The mixture was filtered off and the filtrate was discarded. The retained orange precipitate of lutein was washed with distilled water until the filtrate was almost colorless and the pH was neutral. The precipitate was then washed sequentially with cold (0° C.-5° C.) ethanol (200 mL) and hexane (200 mL), respectively. The yield of lutein obtained as orange crystals from three experiments employing different ratios of water / ethanol are shown below. The lutein obtained in all three experiments was shown to be about 70% pure by spectrophotometric analysis.
[0034]
Weight (g) of Crude LuteinIsolated from 200 g Saponi-Ratio of Water / Ethanolfied Marigold Oleoresin2.3 / 1283.0 / 1221.0 / 215
[0035]This example demonstrates the highest yield of lutein is obtained with 2.3 / 1 ratio of water / ethanol. The final purification step by recrystall...
example 3
Preparation of Lutein Dose For Oral Supplementation
[0044]Carotenoids are considered among the fat soluble nutrients and are usually associated with the lipoprotein fractions of human blood. Therefore, the absorption and bioavailability of carotenoids is significantly increased if these compounds are orally ingested with a small amount of an oil or foods that contain certain amounts of lipids. In a human study that was conducted the following preparation of lutein resulted in excellent absorption of this compound by the subjects as determined from the analysis of their plasma carotenoid profile. In the following, the procedure employed for the preparation of a small batch (100 dose, each 10 mg) of purified lutein is described.
[0045]Purified lutein from marigold flowers extract (1 g) was added to absolute alcohol (75 mL). To this solution α-tocopherol (50 mg) and food grade polysorbate 80 (an emulsifier, 4 g) was added and the mixture was sonicated for 10 minutes. The addition of α-to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com