Diaryl amidine compound, its production, medicinal composition and use
A diaryl amidine compound technology, applied in the field of new diaryl amidine compounds, can solve the problem of hysteresis of onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
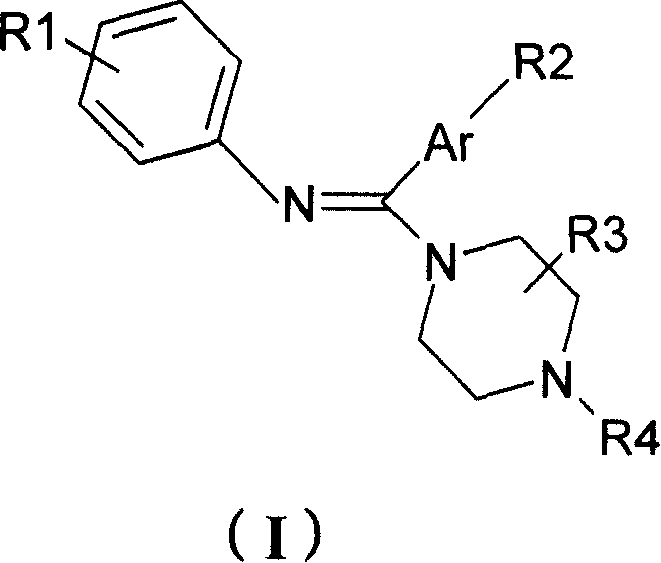
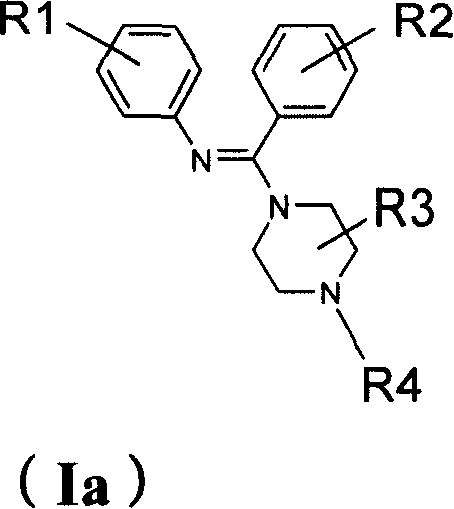
[0143] Preparation of 1-(N-phenylaminobenzyl)piperazine (compound 001)
[0144] Add 1.0 g (5.1 mmol) of N-phenylbenzamide and 2 ml of benzene into the reaction flask, heat at 50°C for 30 minutes, then add 1.1 g (5.3 mmol) of phosphorus pentachloride in batches, and stir at 90°C for 2 hours , the solid is completely dissolved. Benzene and phosphorus oxychloride were evaporated under reduced pressure, and the residue was cooled to room temperature. A solution of 0.9 g (10.5 mmol) of anhydrous piperazine dissolved in 20 ml of benzene was added in one portion, and stirred at room temperature for 2 hours. Leave overnight at room temperature. After filtering, the solid was washed with acetone, and the filtrate and washings were combined and concentrated under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel chromatography, eluted with petroleum ether / ethyl acetate / triethylamine (100:50:1), detected by thin-layer silica gel, and...
Embodiment 2
[0148] Preparation of 1-[N-(4'-trifluoromethylphenyl)aminobenzyl]piperazine (Compound 002)
[0149] a) Preparation of N-(4-trifluoromethylphenyl) benzamide
[0150] Add 12ml of 4-trifluoromethylaniline (15.4 grams, 0.099mol) in the reaction flask, cool in an ice bath, add dropwise 10ml of benzoyl chloride (12.1 grams, 0.086mol) and 60ml of 10% aqueous sodium hydroxide solution while stirring, always Keep the reaction solution alkaline. After the addition was complete, stirring was continued at room temperature for 1 hour. The precipitated solid was collected by filtration and recrystallized from ethanol to obtain 19 g of white crystals (83% yield), mp 203-204°C.
[0151] b) Preparation of 1-[N-(4'-trifluoromethylphenyl)iminobenzyl]piperazine (compound 002) Add a) obtained N-(4-trifluoromethylphenyl)benzene into the reaction flask 1.0 g (3.8 mmol) of formamide and 2 ml of benzene were heated at 50°C for 30 minutes, then 0.85 g (4.0 mmol) of phosphorus pentachloride was added...
Embodiment 3
[0155] Preparation of 1-[N-(3'-methylphenyl)iminobenzyl]piperazine hydrochloride (compound 003)
[0156] a) Preparation of N-(3-methylphenyl)benzamide
[0157] Add 5.0 grams (46.7 mmol) of 3-methylaniline and 12 ml of dichloromethane to the reaction flask, cool in an ice bath, and add dropwise a solution of 5.4 ml (6.6 grams, 46.7 mmol) of benzoyl chloride dissolved in 20 ml of dichloromethane while stirring. and 30ml of 10% sodium hydroxide aqueous solution, and keep the reaction liquid alkaline all the time. After the addition is complete, continue to stir and react at room temperature for 1 hour. The precipitated solid was collected by filtration, washed with saturated aqueous sodium bicarbonate and water, dried, and recrystallized from ethanol to obtain 8.02 g of white crystals, mp 122-124°C, yield 81.3%.
[0158] b) Preparation of 1-[N-(3'-methylphenyl)iminobenzyl]piperazine hydrochloride (compound 003)
[0159] Add 2.0 g (9.47 mmol) of N-(3-methylphenyl) benzamide obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com