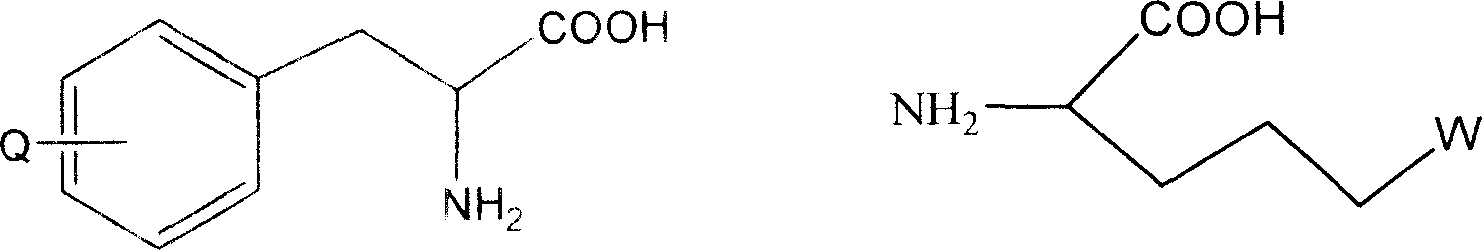Novel LHRH antagonist
A CH2, R2N technology, applied in the field of decapeptide derivatives, can solve the problems of short half-life, limited application of LHRH antagonists, and high release of histamine, and achieve the effect of good antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0237] The preparation method of the compound of the present invention
[0238] The preparation of the compound of the present invention can adopt the solid-phase synthesis method, with MBHA resin as the carrier, Boc-protection strategy, DCC / HOBT or BOP / DIEA as the condensation reagent, HCl / dioxane as the deprotection reagent, after the reaction, the liquid HF cleaves decapeptide derivatives from MBHA resin. Boc-protected amino acids can be purchased from the market, or synthesized according to the methods in the Examples or methods known in the art. The compounds of the present invention may also be prepared by other methods known in the art.
[0239] In another aspect, the present invention also relates to intermediates useful in the preparation of the compounds of the present invention and processes for their preparation.
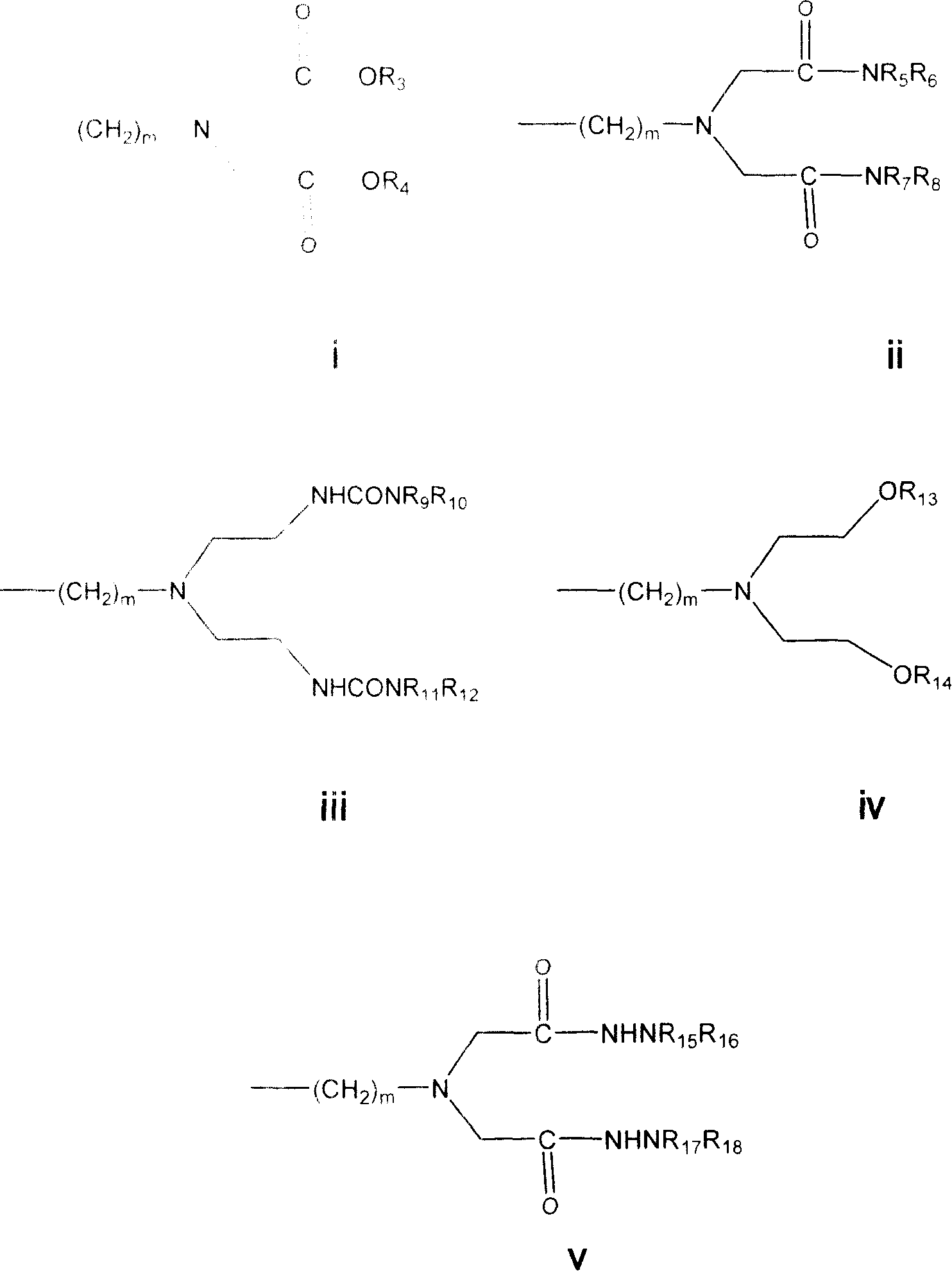
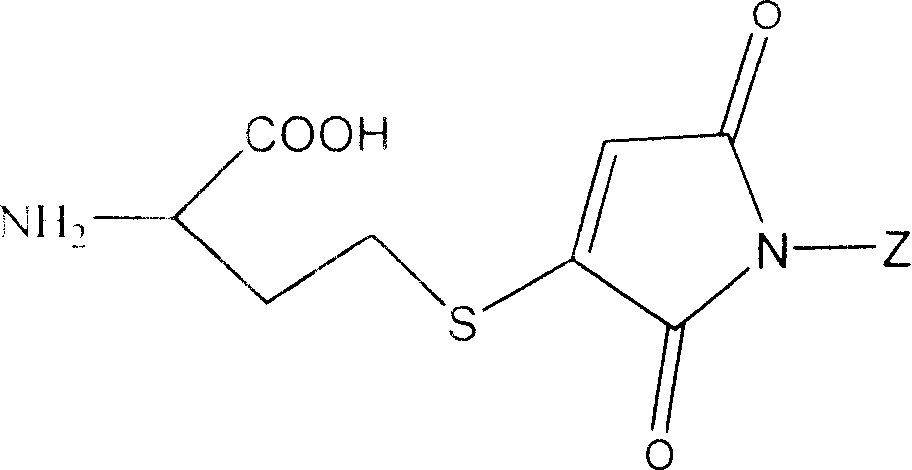
[0240] In one embodiment the invention relates to Boc-p-CH 2 Cl-Phe and its preparation method.
[0241] In another embodiment the invention relates...
Embodiment 1
[0258] Example 1: Boc-p-CH 2 Synthesis of Cl-Phe
[0259] Ac-Phe(p-CH 2 Cl)-Phe-OEt (prepared in this laboratory according to a conventional method, 5g, 17.6mmol) was refluxed in 40mL concentrated hydrochloric acid and 100mL dioxane for 10 hours, and then the hydrochloric acid and dioxane were evaporated to obtain a solid without separation and purification Add 50mL of methanol directly under the ice bath, adjust the pH to 9 with TEA, then add 35.2mmol (3.9mL), and then add 4.6g (Boc) 2 O (21.1 mmol), stirred at room temperature for 12 hours, spin off methanol, adjust the pH of the aqueous solution to acidic, extract with ethyl acetate, wash the ester layer twice with water, and dry over anhydrous sodium sulfate. The ester layer was concentrated, and the oil was recrystallized from ethyl acetate-petroleum ether after column chromatography to obtain 2.7 g of white crystals, with a total yield of 49.1%. TLC detection: chloroform:methanol:HOAc (20:1:0.5), Rf=0.6.
Embodiment 2
[0260] Embodiment 2: Boc-Phe (NA B M) synthesis
[0261] Boc-p-CH 2Cl-Phe (prepared as in Example 1) (1.75g, 5mmol) and dibenzyl iminodiacetate (1.88g, 6mmol, synthesized according to the method described in the following references: Huang Weide, Chen Changqing, Polypeptide Synthesis, Science Publishing Society, 1985, p47) was placed in a 100ml round bottom flask, and 50mL of ethanol was added to dissolve it. After adding TEA (1.69mL, 12mmol) in an ice bath, stir at room temperature for 72 hours, spin off ethanol, adjust the pH of the aqueous solution to alkaline, wash with ether and then adjust the pH of the aqueous phase to acidic, extract with ethyl acetate, wash twice with water, and The layer was dried over anhydrous sodium sulfate. The ester layer was concentrated, and the solid was recrystallized from ethyl acetate-petroleum ether to obtain 1.80 g of white crystals, with a yield of 61%. TLC detection: chloroform:methanol:HOAc (20:1:0.5), Rf=0.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com