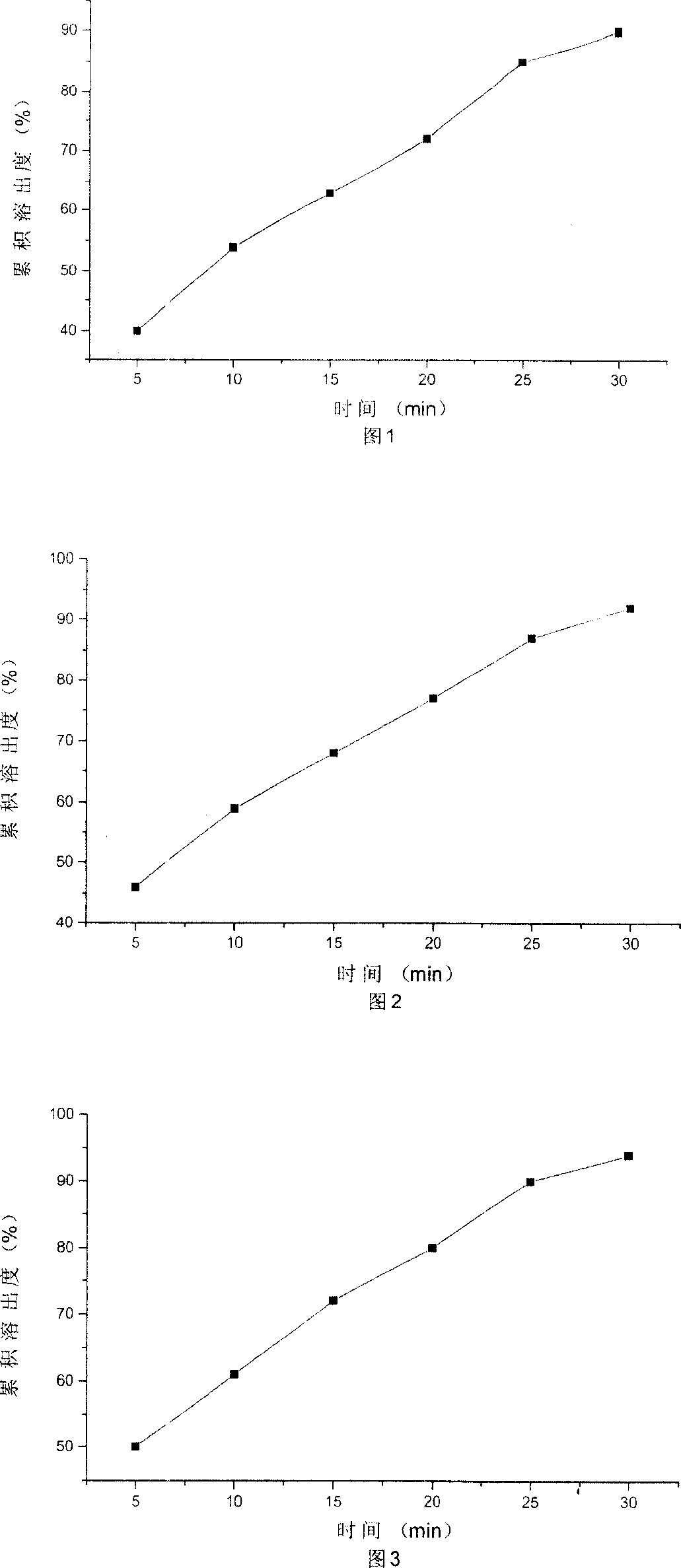
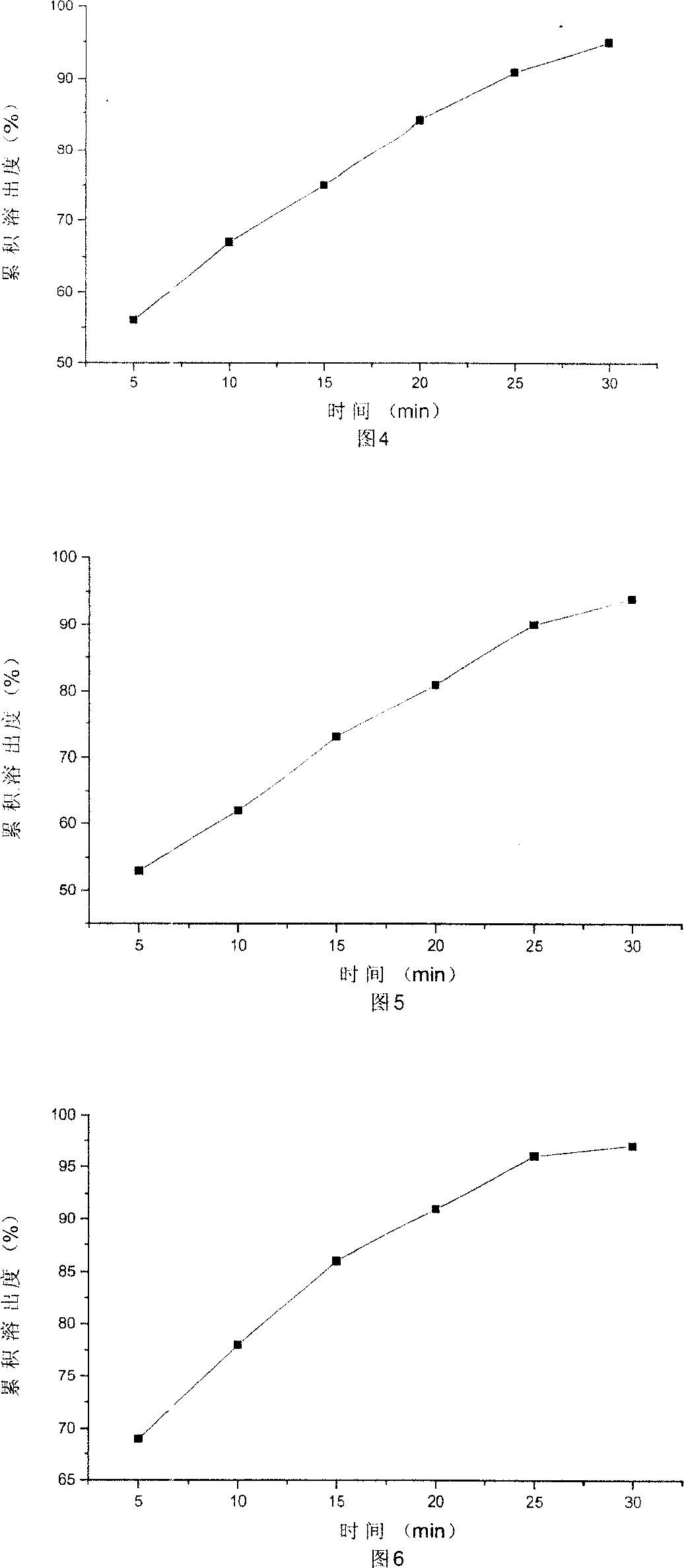
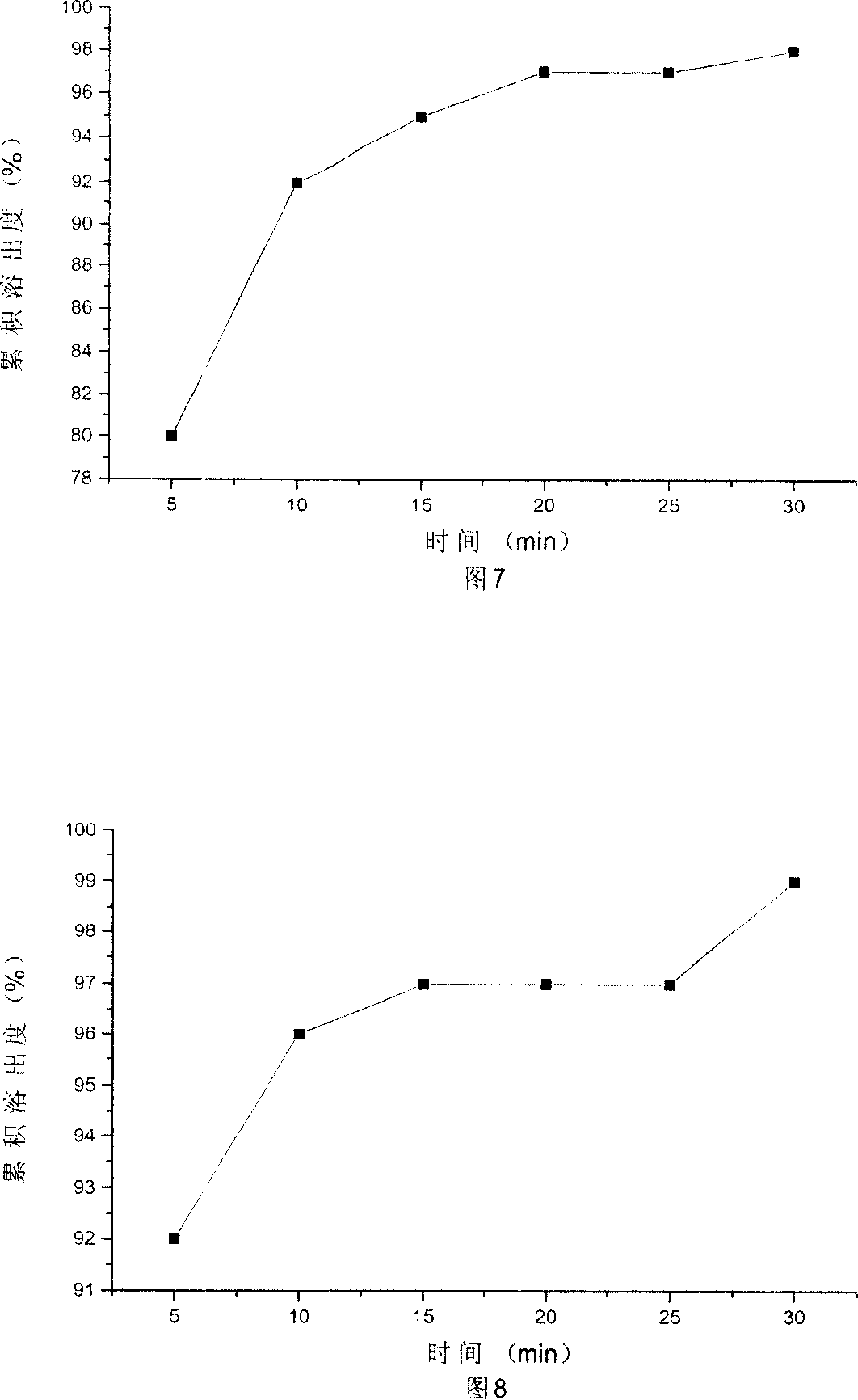
Dispersible tablet of alfuzosin hydrochloride
A technology of alfuzosin hydrochloride and dispersible tablets, which is applied in the direction of organic active ingredients, medical preparations containing active ingredients, pill delivery, etc., can solve the problems of cumbersome blood drug concentration measurement, difficulty in implementation, and difficulty in popularization, and achieve Avoid orthostatic hypotension, reduce adverse reactions, and take convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0027] Embodiment (prescription quantity is 1000):
[0028] Eight specific prescriptions:
[0029] Prescription A:
[0030] Alfuzosin Hydrochloride 0.1g
[0031] Microcrystalline cellulose (filler) 48g
[0032] Sodium carboxymethyl starch (disintegrant) 0.5g
[0033] Magnesium stearate (lubricant) 0.9g
[0034] Micropowder silica gel (glidant) 0.5g
[0035] Preparation process: the main drug passes through a 120-mesh sieve, the filler and disintegrant pass through a 100-mesh sieve, weigh the prescribed amount of filler and disintegrant and mix evenly, and then mix the prescribed amount of the main drug with it in an equal-volume incremental method , add the prescribed amount of lubricant, mix well, and press into tablets. Dispersible tablet dispersion time limit is 3.0min.
[0036] Prescription B:
[0037] Alfuzosin Hydrochloride 1g
[0038] Microcrystalline cellulose (filler) 80g
[0039] Hydroxypropylcellulose (disintegrant) 3g
[0040] 2% PVP k30 Absolute ethano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com