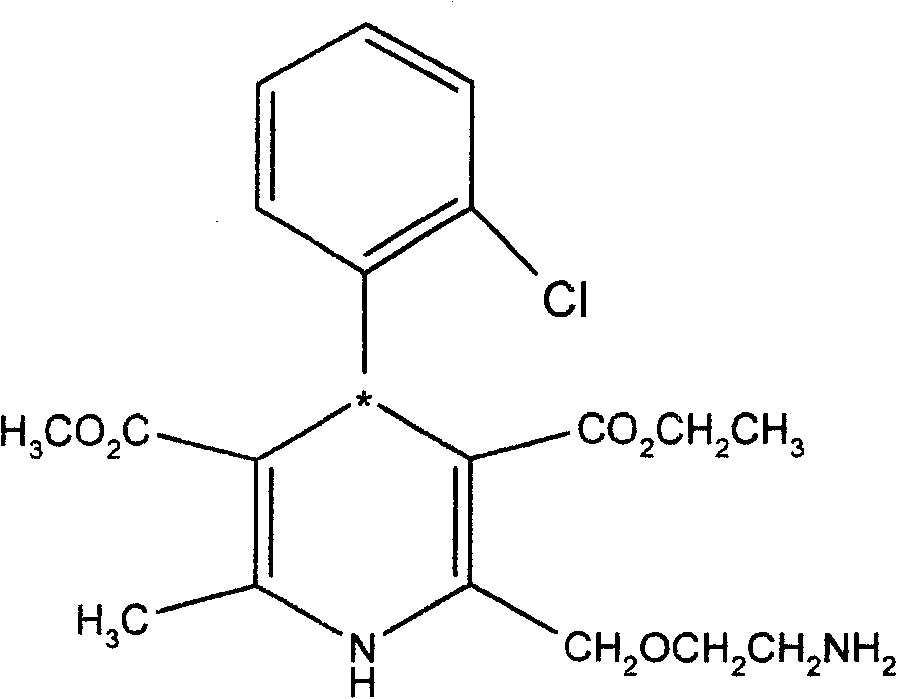
Resolution method of optically active amlodipine
A kind of amlodipine and peaceful technology, applied in the splitting field of optically active amlodipine, can solve the problems of long reaction time, increased equipment cost, unsuitable for large-scale production and the like, and achieves the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment one methyl ethyl sulfoxide
[0024] 1. Take four 1000ml reaction bottles (with thermometers and condenser tubes), first add 350ml of acetone, 2mol of methyl ethyl ether, put the reaction bottle in an ice bath, add 1.98mol of hydrogen peroxide (concentration is 31%) dropwise, and the temperature should be controlled at Below 40°C, keep warm at 25-35°C for 6 hours after the addition, and remove excess methyl ethyl sulfide, acetone and water under vacuum at 60°C to obtain 176 grams of product with a water content of 5.2% and a yield of 91%. . Gas chromatography purity 99.2%.
[0025] 2. Take 80 grams of the above product (water content 5.2%), add 60 grams of calcium oxide, heat up to 70 degrees, dry for 5 hours, filter after cooling down to room temperature, filter the filtrate under reduced pressure, and vacuum distill to obtain 62 grams of fractions. It is 81%, moisture content is 0.4%, gas chromatography purity is 99.3%.
Embodiment 2
[0026] The preparation of embodiment two (S)-(-)-amlodipine
[0027] 5 grams (0.012mol) of amlodipine is dissolved in 40ml water content and is in the methyl ethyl sulfoxide (made by embodiment 1) of 0.4%, add and dissolve 1.0 grams (0.006mol) D-(-)-tartaric acid 40ml of methyl ethyl sulfoxide solution with a water content of 0.5%, stirred at room temperature for 1 hour, precipitated, filtered, washed with 15ml of acetone, and vacuumed at 50°C for 4 hours to obtain (S)-(-)-amlodipine, D- (-)-tartrate, methyl ethyl sulfoxide three-component solvate 2.40 grams.
[0028] Add 2.40 g of the above-mentioned solvate after drying, add 35 ml of dichloromethane, 18 ml of 2N sodium hydroxide solution, stir and react for 30 minutes, let stand, separate the organic layer, add an appropriate amount of anhydrous sodium carbonate to dry, filter, and use a small amount of dichloro Wash the filter cake with methane, concentrate the filtrate under reduced pressure, add an appropriate amount of ...
Embodiment 3
[0029] The preparation of embodiment three (S)-(-)-amlodipine
[0030] Using undried methyl ethyl sulfoxide in Example 1, with a water content of 5.2%, the others are the same as in Example 2, to obtain (S)-(-)-amlodipine, D-(-)-tartrate, methyl ethyl sulfoxide three 2.35 g of solvate composed of components, and finally 1.49 g of (S)-(-)-amlodipine was obtained. The enantiomeric excess value (ee) determined by chiral column HPLC was 98.5%, and the total yield was 59.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com