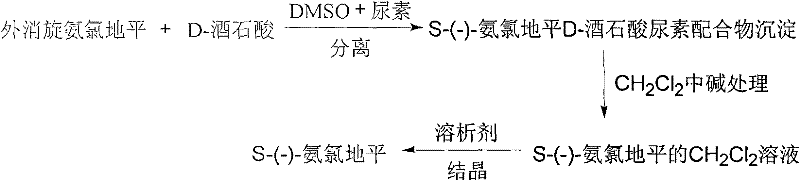
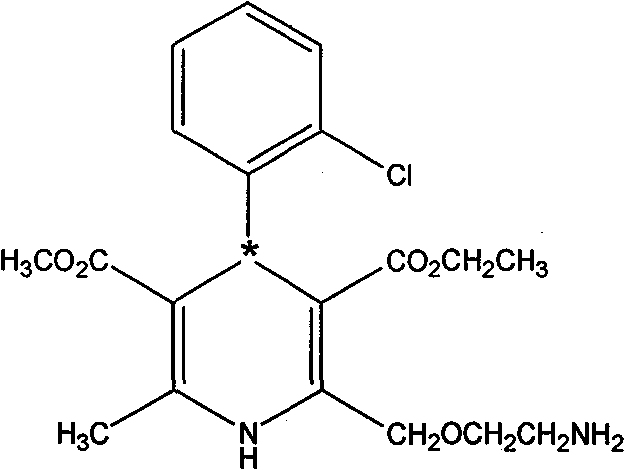
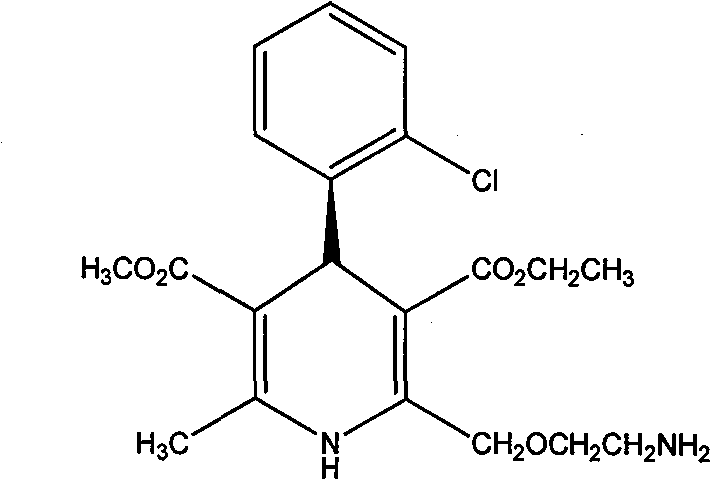
A method for splitting and obtaining s-(-)-amlodipine
A technology of amlodipine and dichloromethane, which is applied in the field of optical separation to obtain S--amlodipine, can solve problems such as pollution, surplus, and difficulty in reaching clinical medical standards, and achieve simple preparation process and shortened reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve 1.0 g of racemic amlodipine in 10 mL of dimethyl sulfoxide, then add it to 10 mL of dimethyl sulfoxide solution containing 0.1847 g of D-tartaric acid, and finally, add 3 mL of urea with a concentration of 0.0247 g / mL aqueous solution. Heat up to 50°C and stir, after 5-10min precipitation appears, adjust to 10°C after half an hour and continue to stir for 16h, after the reaction is completed, put the reaction system in the refrigerator for 10h, filter, rinse with methanol and recrystallize, then vacuum dry After 4 hours, 0.4894 g of a white solid was obtained, with a yield of 97.88%. The e.e% was 92.8% when detected by a chiral chromatographic column.
[0040] Add 0.4894g of the above-mentioned solvate after drying, add 40mL of dichloromethane, 30mL of 2N sodium hydroxide solution, stir and react for 30 minutes, let it stand, separate the organic layer, add an appropriate amount of anhydrous sodium carbonate to dry, filter, and use a small amount of dichloro W...
Embodiment 2
[0042] Dissolve 10.0 g of racemic amlodipine in 150 mL of dimethyl sulfoxide, then add it to 150 mL of dimethyl sulfoxide containing 0.923 g of D-tartaric acid, and finally add 30 mL of 0.01 g / mL Aqueous urea solution. Heat up to 50°C and stir. After 5-10 minutes, precipitation appears and continue to stir for half an hour. Then adjust to 40°C and continue to stir for 3 hours. After the reaction, place in the refrigerator for 10 hours, vacuum filter, and rinse with dimethyl sulfoxide for recrystallization Afterwards, vacuum-dried for 4 hours to obtain 4.271 g of a white solid with a yield of 85.42%. The e.e% was 94.3% when detected by a chiral chromatographic column.
[0043]Add 3.871 g of the above-mentioned solvate after drying, add 155 mL of dichloromethane, 310 mL of 2N sodium hydroxide solution, stir and react for 30 minutes, let it stand, separate the organic layer, add an appropriate amount of anhydrous sodium carbonate to dry, filter, and use a small amount of dichloro...
Embodiment 3
[0045] Dissolve 10.0 g of racemic amlodipine in 50 mL of dimethyl sulfoxide, then add it to 50 mL of dimethyl sulfoxide solution containing 3.7 g of D-tartaric acid, and finally add 60.7 mL of it to a concentration of 0.0247 g / mL urea aqueous solution. Heat to 50°C and stir, 5-10 minutes later precipitation appears, adjust to 60°C after half an hour and continue to stir for 24 hours, place in the refrigerator for 10 hours, centrifuge to obtain a precipitate, rinse with the original co-solvent for recrystallization, and vacuum dry for 4 hours to obtain a white solid 4.318g, the yield is 86.36%, detected by chiral chromatography column, e.e% is 96.2%.
[0046] Add 4.318 grams of the above-mentioned solvate after drying, add 43.2 mL of dichloromethane, 43.2 mL of 2N sodium hydroxide solution, stir for 30 minutes, let stand, separate the organic layer, add an appropriate amount of anhydrous sodium carbonate to dry, filter, and use a small amount of The filter cake was washed with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com