Application of Schizandrin A on curing drug resistance for multiple drugs of treating tumors
A schisandrin A, multi-drug resistance technology, applied in the direction of antitumor drugs, drug combinations, ether/aldol active ingredients, etc., can solve the toxic and side effects, cardiovascular toxicity, and unsatisfactory effect of solid tumor resistance reversal and other problems, to achieve a significant dose-dependent, strong reversal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Take KB, KBv200, MCF-7, MCF-7 / Adr, Bel in the logarithmic growth phase 7402 The cells were seeded in a 96-well culture plate (Coster), and after 24 hours of culture, gradient concentrations of anticancer drugs, reversal agents and control solvents were added. Continue to cultivate for 72 hours, then discard the culture medium, add 0.5 mg / ml thiazolium (MTT) 100 μl (diluted with RPMI 1640 culture medium without serum) to each well, continue to cultivate for 4 hours, discard the thiazolium, add to each well 150 μl of dimethyl sulfoxide was shaken slightly to dissolve the precipitate, and the absorbance was measured at 570 nm in a microplate reader (Bio-Rad 450 type). Set up three to four replicate wells in each group and take the average value to calculate the IC 50 . 10 μmol / L verapamil was used as a positive control. See Table 1-10 for specific results.
[0043] Table 1. Effect of Schisandrin A on the sensitivity of KBv200 cells to vincristine
[0044]
[0045] ...
Embodiment 2
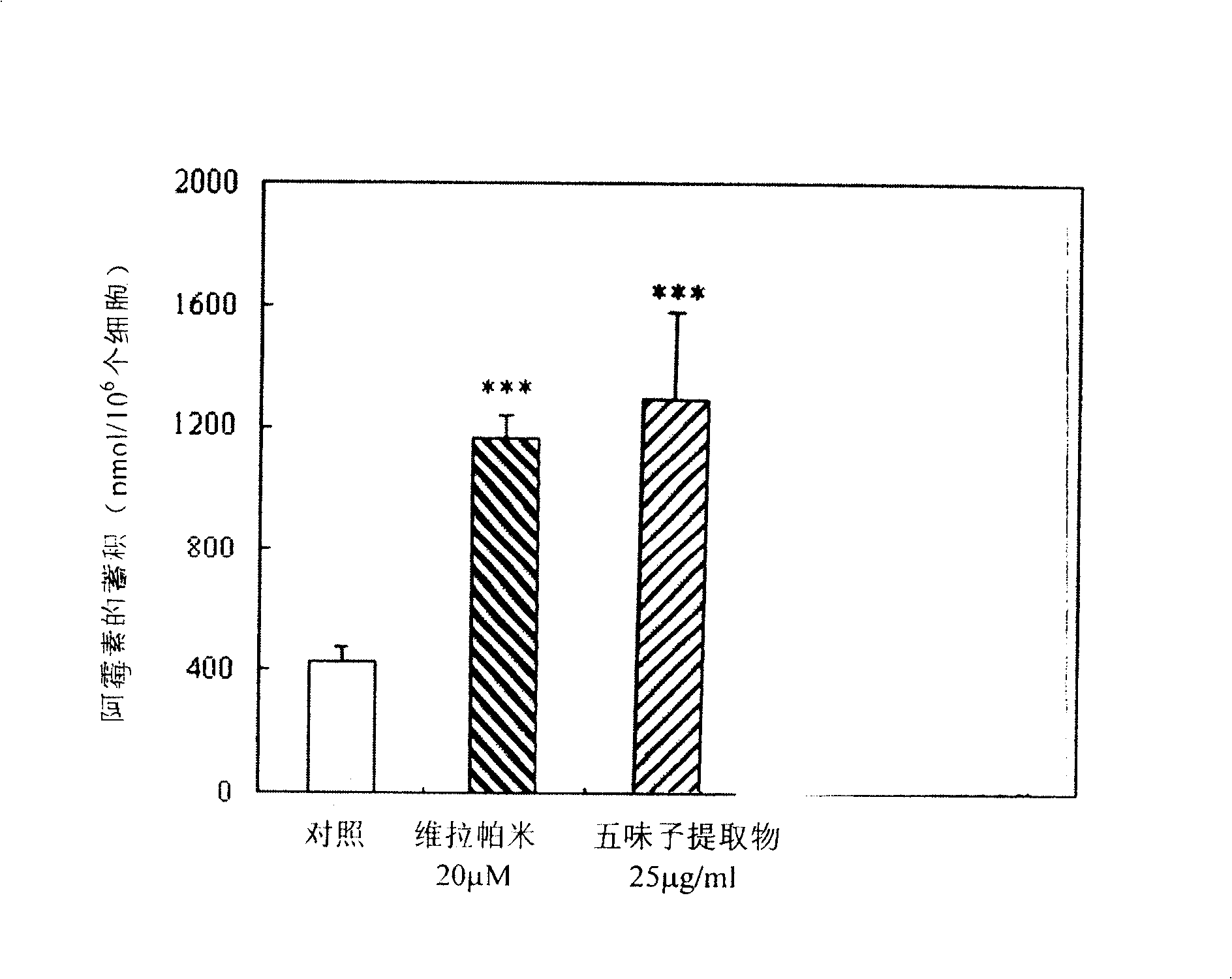
[0066] Take the logarithmic growth period Bel 7402 Cells, when the cells cover 70-80% of the bottom of the bottle, replace the RPMI 1640 culture medium containing 10 μmol / L doxorubicin, and add schisandrin at the final concentrations of 50 and 25 μmol / L respectively, and add 10 μmol / L vera Pamir was used as a positive control, and three parallel samples were set up for each group, at 37°C, 5% CO 2 Continue to incubate for 3 hours, collect the cells after washing three times with ice-cold PBS (pH 7.4), take a certain number of cells, suspend the cells with 1.2ml hydrochloric acid (0.3N)-ethanol (50%), and break the cells on a sonicator Afterwards, centrifuge at 10,000rpm for 15 minutes, take 1.0ml of the supernatant and add 2.0ml of the above-mentioned hydrochloric acid-ethanol solution, measure the fluorescence value of adriamycin with a fluorescence spectrophotometer, the excitation wavelength is 470nm, and the emission wavelength is 580nm. Content-fluorescence standard curv...
Embodiment 3
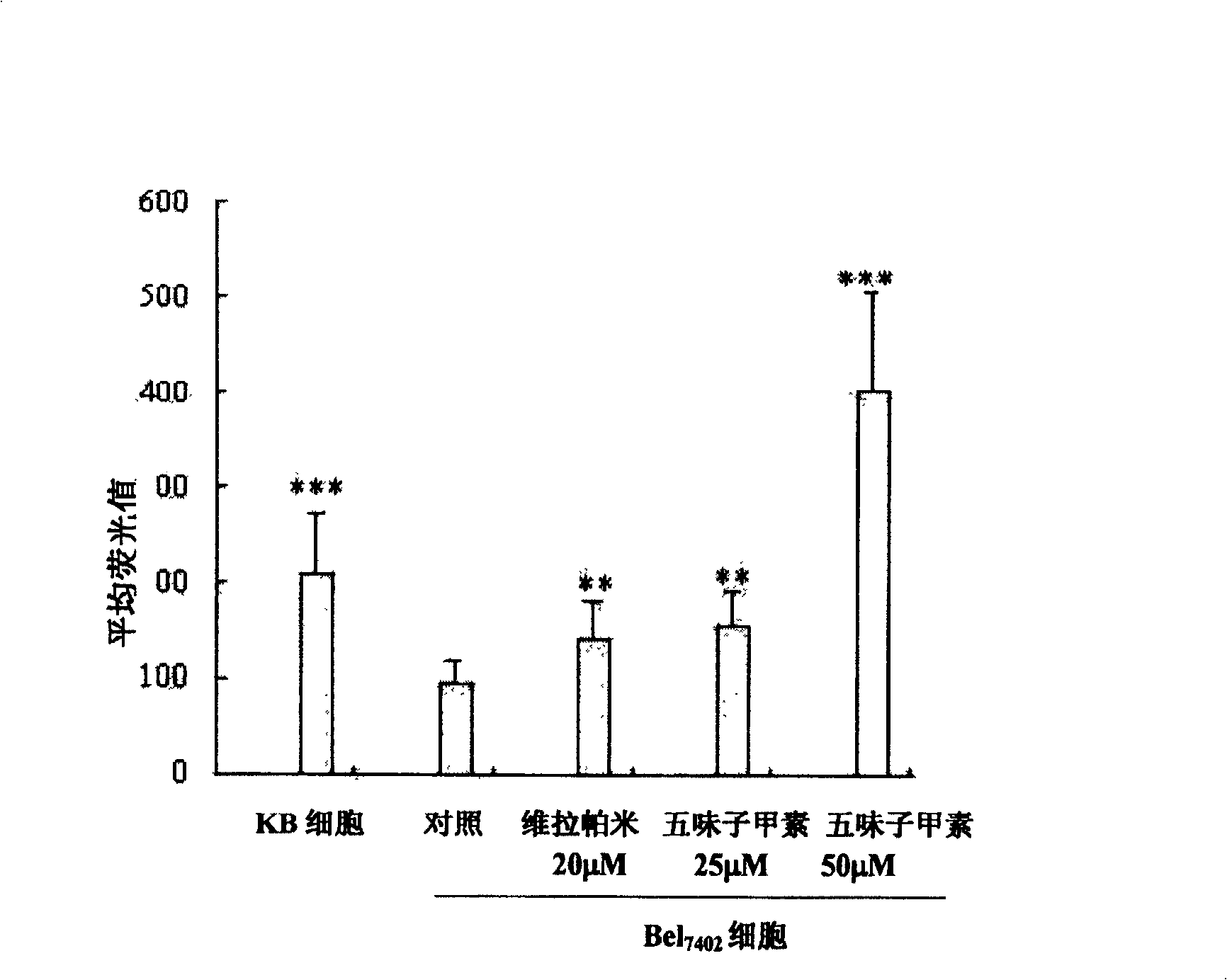
[0068] Inoculate KB, KBv200 and Bel 7402 The cells were placed on a petri dish with a diameter of 3.5 cm. After culturing for 24 hours, the unattached cells were washed away, and rhodamine 123 (final concentration: 250 ng / ml) and / or different concentrations of Schizandrin were added respectively. In the control group, only schisandrin was added. After incubating at room temperature for 30 minutes, the cells were washed twice with 0.1 M phosphate buffer, and the accumulation of rhodamine 123 in the cells was observed by laser scanning confocal microscopy. Five fields of view were randomly selected for each petri dish to observe, and the average fluorescence intensity was analyzed by computer software. At least 50 cells were analyzed per sample. The main parameters of each Petri dish are the same during image processing. see results figure 2 , 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com