Composite particle for electrode, its manufacturing method and secondary battery
A composite particle and manufacturing method technology, applied in secondary batteries, non-aqueous electrolyte storage batteries, battery electrodes, etc., can solve the problems of incomplete construction of electronic conduction network, inability to obtain cycle characteristics, low carbon nanofiber generation rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
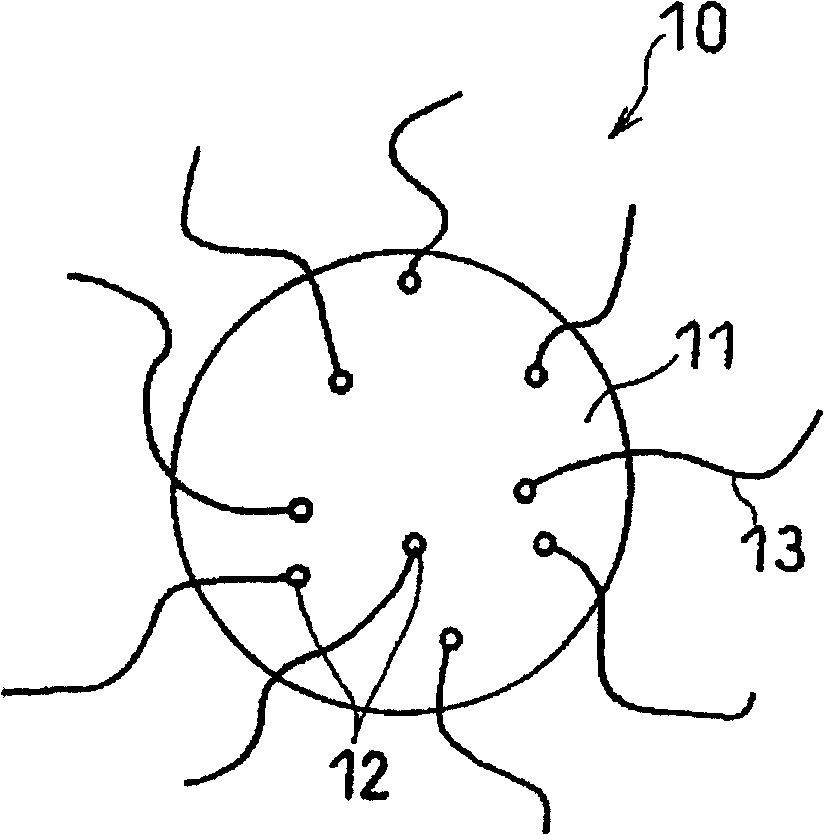
[0166]Dissolve 1 g of nickel nitrate hexahydrate (special grade) produced by Kanto Chemical Co., Ltd. in 100 g of deionized water. The obtained solution was mixed with 100 g of silicon particles (Si) produced by Kosun Chemical Laboratories Co., Ltd. which had been pulverized to a size of 10 μm or less. After stirring the mixture for 1 hour, water was removed with an evaporator apparatus. As a result, active material particles composed of silicon particles as an electrochemically active phase and nickel nitrate supported on the surface were obtained.
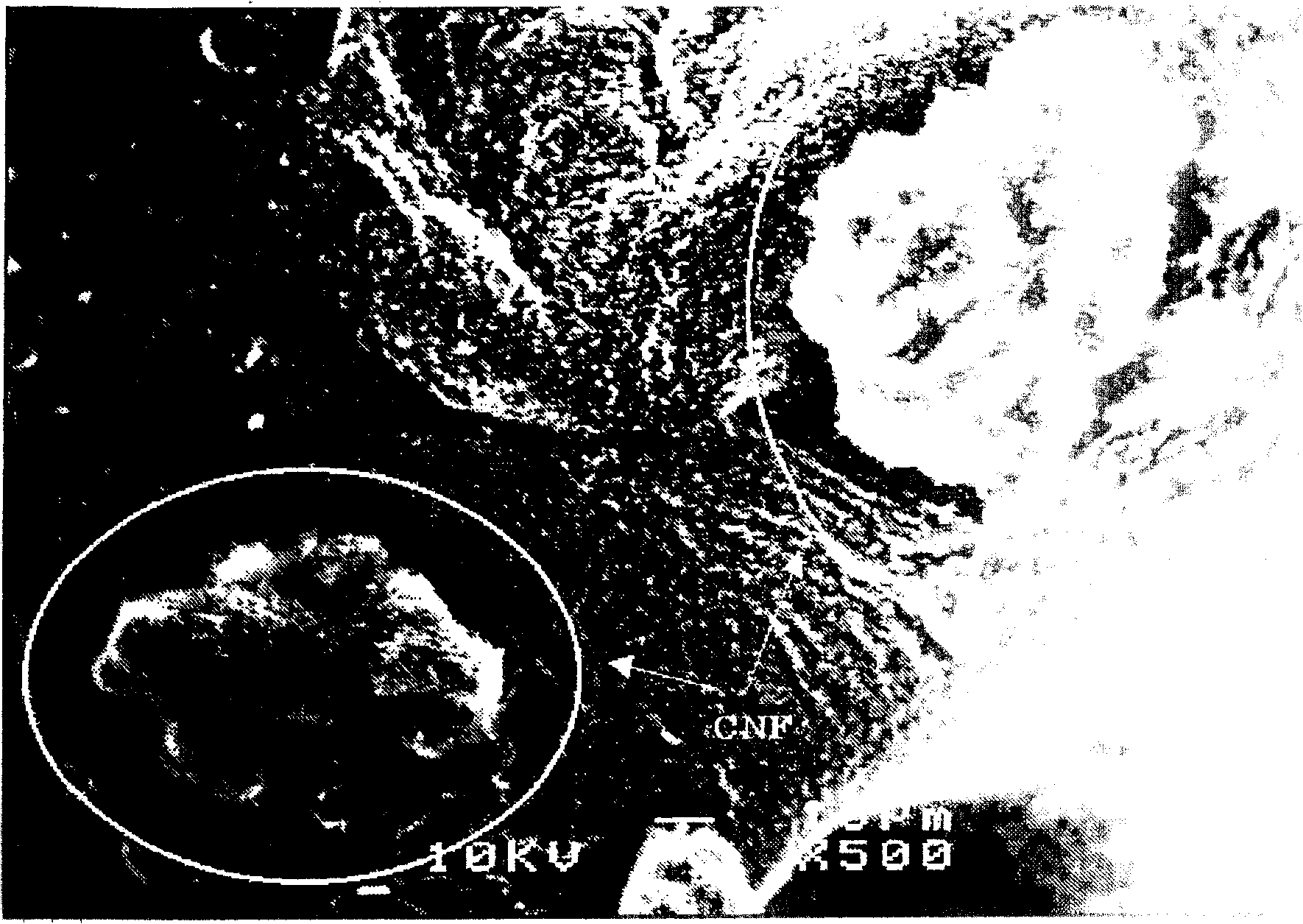
[0167] The silicon particles carrying nickel nitrate were charged into a ceramic reaction vessel, and the temperature was raised to 550° C. in the presence of helium gas. Thereafter, the helium gas was replaced with a mixed gas of 50% by volume of hydrogen gas and 50% by volume of methane gas, and the reaction vessel was kept at 550° C. for 3 hours. As a result, tubular carbon nanofibers with a fiber diameter of about 80 nm and...
Embodiment 2
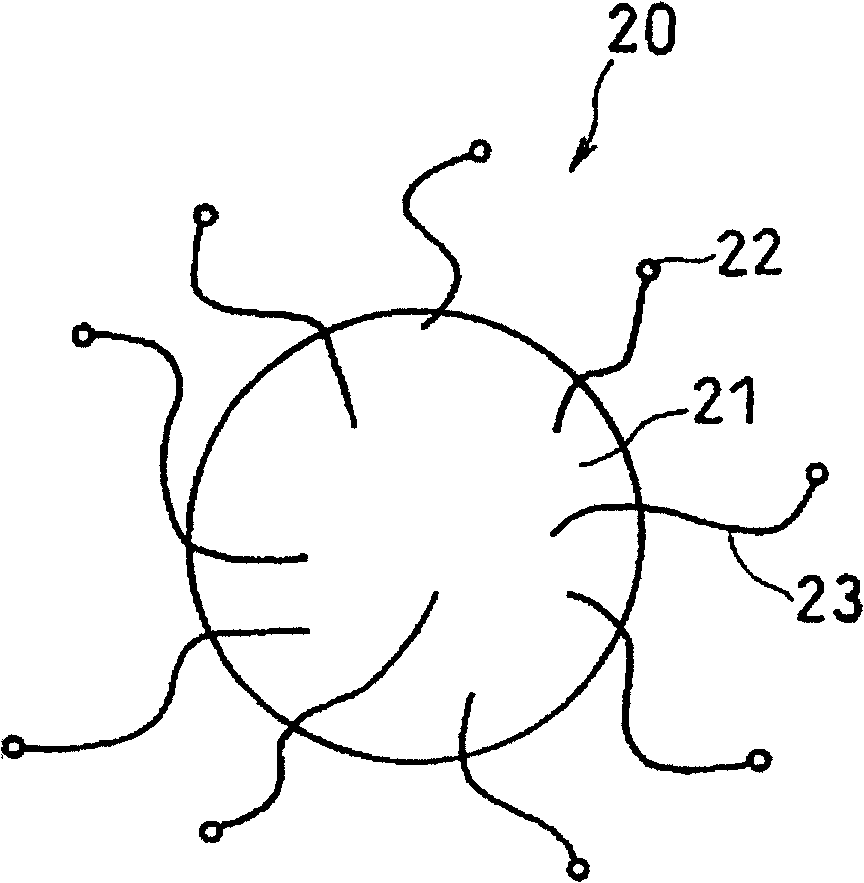
[0172] Cobalt nitrate hexahydrate (special grade) produced by 1g Kanto Chemical Co., Ltd. replaces 1g nickel nitrate hexahydrate and is dissolved in 100g deionized water; Electrode material B for an electrolyte secondary battery. The particle size of the cobalt particles carried on the silicon particles was substantially the same as that of the nickel particles in Example 1. The fiber diameter, fiber length, and weight ratio to the active material particles of the grown herringbone carbon nanofibers were almost the same as in Example 1. Here, in SEM observation, in addition to fibers having a fiber diameter of about 80 nm, the existence of fine fibers having a fiber diameter of 30 nm or less was also confirmed.
Embodiment 3
[0174] 20% by weight of silicon particles ground to 10 μm or less and 80% by weight of nickel particles produced by Kanto Chemical Co., Ltd. ground to 10 μm or less were mixed. A mechanical alloying method is used to apply a shear force to the obtained mixture to obtain NiSi alloy particles with an average particle diameter of 20 μm. Except having used the obtained NiSi alloy particle instead of a silicon particle, it carried out similarly to Example 1, and this was set as the electrode material C of a nonaqueous electrolyte secondary battery. The particle size of the nickel particles carried on the NiSi alloy particles was substantially the same as that of the nickel particles in Example 1. The fiber diameter, fiber length, and weight ratio of the grown tubular carbon nanofibers to the active material particles were almost the same as in Example 1. Here, in SEM observation, in addition to fibers having a fiber diameter of about 80 nm, the existence of fine fibers having a fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com