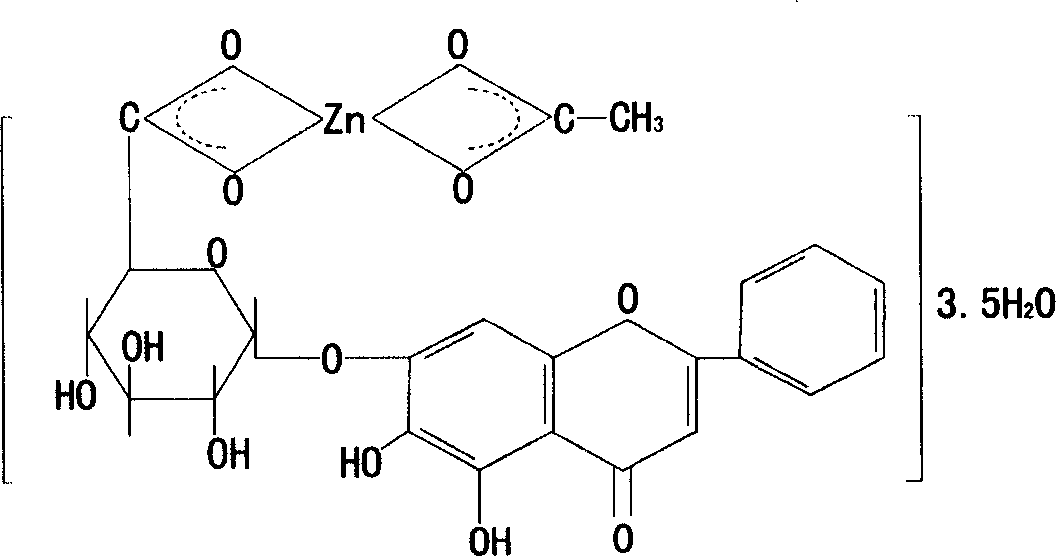
Pharmaceutical preparation containing zinc baicalin
A technology of baicalin zinc and baicalin, which is applied in the field of baicalin zinc pharmaceutical preparations, can solve the problems of drug loss, unsatisfactory drug efficacy and unsatisfactory effects, and achieves prolonged action time, optimal therapeutic effect, and increased bioadhesion. force effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Prepare the following preparation raw materials in parts by weight:
[0033] Sodium alginate 18 parts
[0034] Glycerin 2.4 parts
[0035] Tween-80 1.5 parts
[0036] Zinc baicalin 2.0 parts
[0037] By hand, 12.6 g of sodium alginate was added with H 2 O 100ml~150ml, heat to 85°C to make sodium alginate expand and disperse into a colloidal solution, add glycerin, Tween-80 and zinc baicalin, grind evenly, transfer to a flat glass plate after defoaming, and paint with a push rod A uniform film with a thickness of 1.0 mm is dried at 70° C., sliced, and packaged to obtain a baicalin zinc film preparation. If it is prepared by a film coating machine, it is prepared by manual operation, and the machine is used to coat the film after grinding evenly.
Embodiment 2
[0039] Prepare the following preparation raw materials in parts by weight:
[0040] Agar 10 parts
[0041] Glycerin 1.5 parts
[0042] Tween-80 3.0 parts
[0043] Baicalin zinc 1.5 parts
[0044] By hand, add H to 14.5 g of agar 2 O 120ml~150ml, heat 85℃ to make the agar expand and disperse into a colloidal solution, add glycerin, Tween-80 and baicalin zinc, grind evenly, transfer to a flat glass plate after defoaming, and use a push rod to coat it to a thickness of 0.7 mm uniform film, dried at 80°C, sliced, and packaged to obtain the baicalin zinc film preparation. If it is prepared by a film coating machine, it is prepared by manual operation, and the machine is used to coat the film after grinding evenly.
Embodiment 3
[0046] Prepare the following preparation raw materials in parts by weight:
[0047] 14 parts of hydroxyethyl cellulose
[0048] Glycerin 3.0 parts
[0049] Tween-80 4.0 parts
[0050] Zinc baicalin 3.0 parts
[0051] By hand, add H to 16.0 g of hydroxyethyl cellulose (HEC) 2 O 150ml~180ml, heat 85°C to make hydroxyethyl cellulose (HEC) expand and disperse into a colloidal solution, add glycerin, Tween-80 and baicalin zinc, grind evenly, transfer to a flat glass plate after defoaming, and use The push rod is coated into a uniform film with a thickness of 0.3 mm, dried at 90° C., sliced, and packaged to obtain the baicalin zinc film preparation. If it is prepared by a film coating machine, it is prepared by manual operation, and the machine is used to coat the film after grinding evenly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com