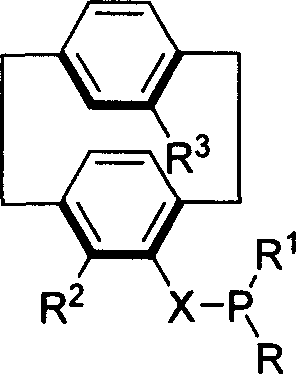
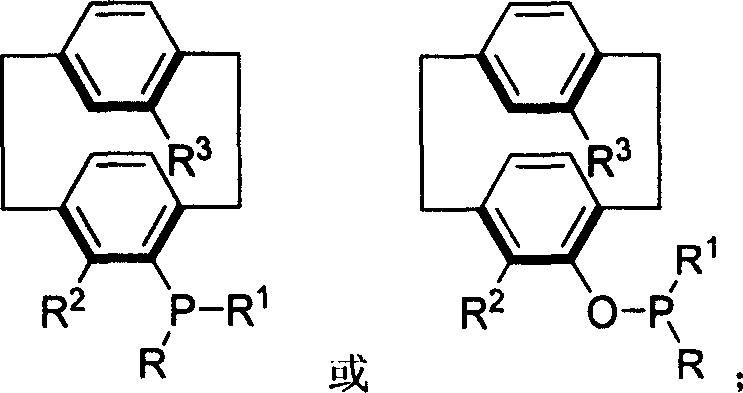
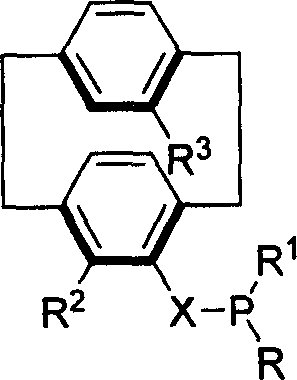
Phosphine compound of possessing plane chirality cyclophane alkyl, synthetic method, and appliction
A phosphine compound and compound technology are applied in the field of phosphine compounds and synthesis of cyclic aryl alkanes, and can solve the problems of difficult preparation, complex structure, easy oxidation and the like of planar chiral cyclo alkane compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Synthesis of planar chiral cycloaryl alkane monophosphine compound
[0025] At 0°C, add (3.444g, 12mmol) 4-bromo[2.2]p-cycloarane to the reaction flask, dissolve it in 150mL of ether, add butyllithium, stir for 1 hour, then add dihydrocarbylphosphine chloride, dihydrocarbyloxy Phosphorus chloride (take diphenylphosphine chloride as an example) (3.5ml, 18mmol), rise to room temperature and react for 8 hours, add 10mL methanol to quench, remove solvent under reduced pressure, silica gel column chromatography (petroleum ether: ethyl acetate = 15 : 1), to obtain the corresponding racemic cycloaryl alkane monophosphine compound H1a 3.669g, yield: 78%. Racemic H1a (0.392g, 1mmol) reacted with cyclopalladium A (0.339g, 0.5mmol) in 10mL of methanol for 0.5 hours, and was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 3: 1) to obtain two non-pairs Enantiomers, reacting them with a dissociation reagent (0.5 mmol recommended sodium p...
Embodiment 2
[0068] Example 2: Synthesis of planar chiral ring aralkoxa monophosphine compound
[0069] With reference to literature (Cram, D.J.; Day, A.C.J.Org Chem.1966,31,1227), in a 250ml three-necked flask, add 4-bromo[2.2]paracyclic arane (3.444g, 12.0mmol), diethyl ether (150ml) , cooled to 0°C, added butyllithium (15ml, 24mmol), stirred at room temperature for 20 minutes, a light yellow solid precipitated, cooled to 0°C again, added freshly distilled trimethyl borate (2.7ml, 24mmol), stirred at room temperature for 1 After one hour, add 0.5M aqueous sodium hydroxide solution (6ml, 3mmol), 30% hydrogen peroxide aqueous solution (4.5ml, 45mmol), heat up to 40°C for 3 hours, cool to room temperature, add 30ml of water, 100ml of dichloromethane, and separate , the solvent was removed under reduced pressure, and purified by column chromatography (dichloromethane) to obtain the known compound 4-hydroxy[2.2]paracyclophane.
[0070] Add 4-hydroxy[2.2]p-cycloarane (202mg, 0.90mmol), toluen...
Embodiment 3
[0083] Embodiment 3: Application in asymmetric allyl amination reaction
[0084] For general operation, take 2-(acetoxy-p-nitrobenzyl)methyl acrylate as an example. Under the protection of argon, the raw material (0.5mmol), phthalimide (1.0mmol) and chiral catalyst (0.01mmol) were dissolved in 2ml of tetrahydrofuran, followed by spotting the reaction after completion of the reaction, and directly separated by silica gel column chromatography to obtain product. If (R)-H2 is used as a catalyst, the yield is 95%, ee(%): 70%.
[0085] 1 H NMR (300MHz, CDCl 3 )δ8.21(d, J=8.9Hz, 2H), 7.87-7.75(m, 4H), 7.61(d, J=9.0Hz, 2H), 6.63(s, 1H), 6.52(s, 1H), 5.66 (s, 1H), 3.72 (s, 3H); EIMS m / z (relative intensity) (%): 367 (M + ,<1), 147(51), 76(100).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative strength | aaaaa | aaaaa |
| Relative strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com