Process for preparing water-soluble polysulfonated naphthylamine
A technology for sulfonated naphthylamine and water-soluble polymer is applied in the field of preparation of polysulfonated naphthylamine, which can solve the problems of tediousness, limited functional application potential of conjugated polymers, low polymer yield and the like, and achieves a simple and efficient synthesis process. , good general applicability, good solution processability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
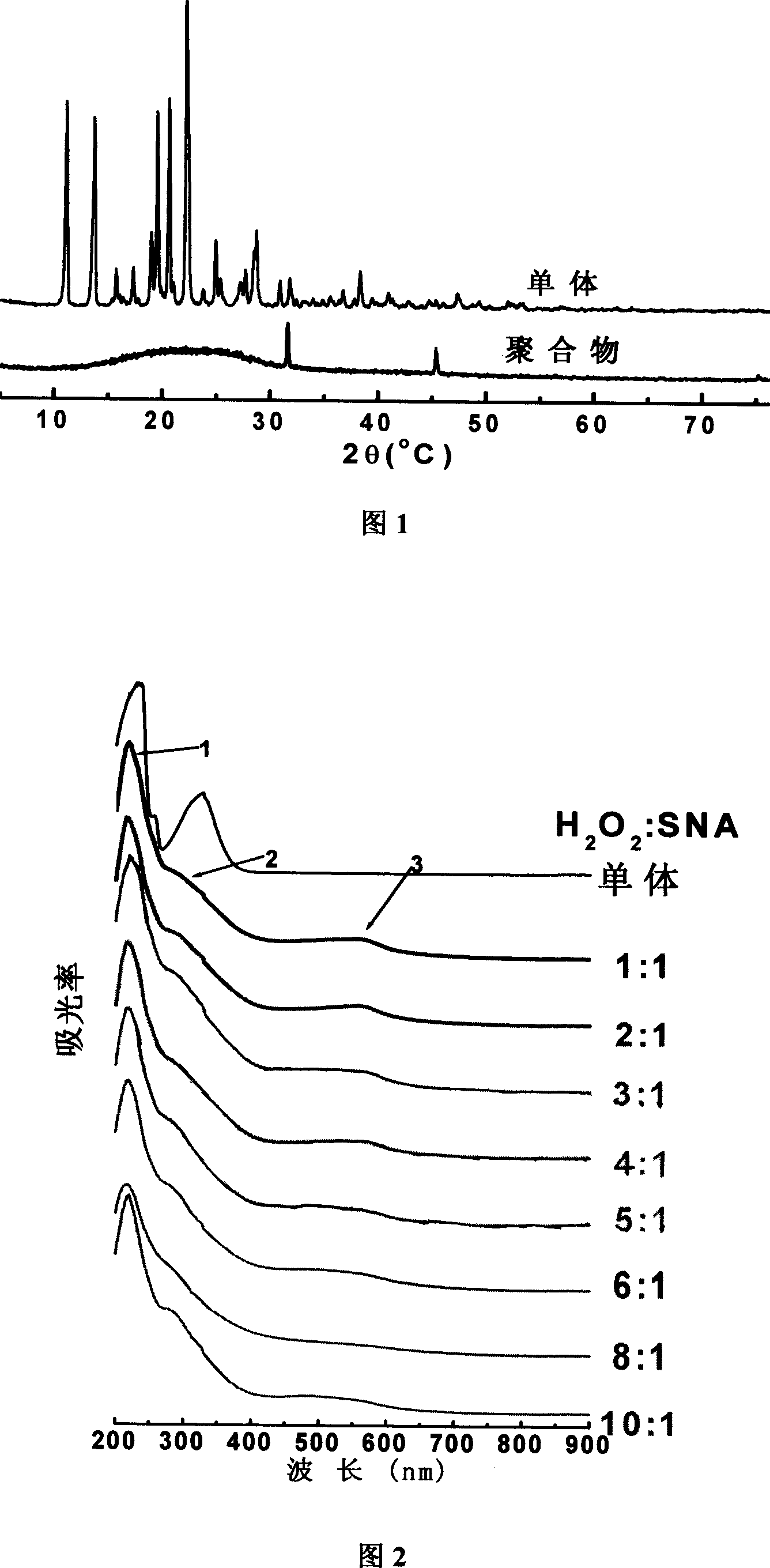
[0022] The following examples will illustrate that the poly(7-sulfonic acid-1-naphthylamine) of the present invention is in an aqueous solution system, adopts hydrogen peroxide / ferrous sulfate redox system to initiate polymerization, and realizes according to the chemical oxidation polymerization reaction path .
[0023] Accurately measure 6mL of water into a 50mL beaker, and then add 2mL of NaOH solution with a concentration of 1mol / L. Accurately weigh 446.5 mg (2 mmol) of 7-sulfonic acid-1-naphthylamine, and transfer it into the above aqueous alkali solution. Stir the blended system with a magnetic stirrer for 18 minutes to completely dissolve the monomer in the aqueous alkali solution, and then add 1 mL (0.05 mmol) of FeSO with a concentration of 50 mmol / L to the beaker. 4 Add 1ml (10mmol) hydrogen peroxide to the solution within about 5 minutes, at this time, the color of the solution changes from light gray to dark brown. The beaker was sealed and the reaction was conti...
Embodiment 2~7
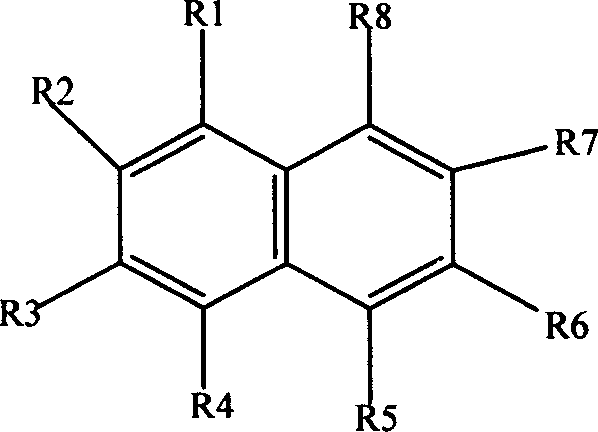
[0025] The following examples will illustrate the influence of different oxygen single ratios on the polymerization yield in the present invention.
[0026] Repeat Example 1, change the addition amount of oxidizing agent hydrogen peroxide, make the ratio of oxygen to single ratio be 1:1, 2:1, 3:1, 4:1, 8:1, 10:1 respectively. A black solid powder was obtained with yields of 15.8%, 21.7%, 23.7%, 25.3%, 87.6%, and 59.5%, respectively. The conductivity measured by tablet method is 2.12×10 -6 S / cm, 2.12×10 -6 S / cm, 2.20×10 -6 S / cm, 2.19×10 -6 S / cm, 1.25×10 -6 S / cm, 0.65×10 -6 S / cm.
Embodiment 8~10
[0028] The following examples illustrate the effect of reaction time on polymer yield in the present invention.
[0029] Example 1 was repeated, and the reaction time was changed so that the reaction time was 6h, 12h, and 28h respectively, and black solid powder was obtained with yields of 44.3%, 52.5%, and 81.4%, respectively. The conductivity measured by tablet method was 1.36×10 -6 S / cm, 1.45×10 -6 S / cm, 2.12×10 -6 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com