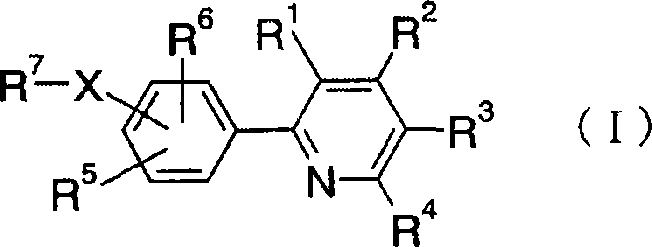
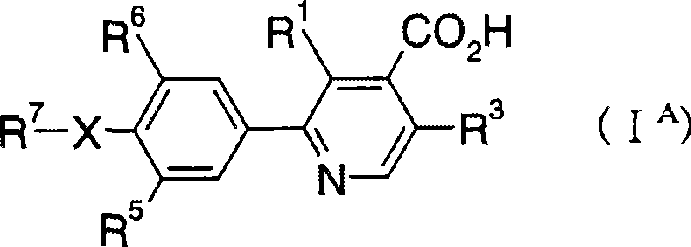
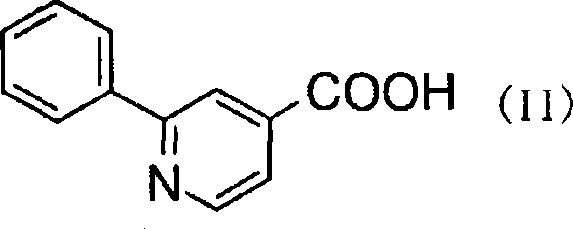
2-phenylpyridine derivative
A technology of phenylpyridine and derivatives, applied in the field of 2-phenylpyridine derivatives, can solve the problems of the inhibition of xanthine oxidase and the inhibition of uric acid synthesis, which is not disclosed or implied, and achieves strong inhibition of xanthine oxidase. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0199] Hereinafter, the production method of the compound (I) of the present invention will be described in more detail based on examples. However, the present invention is not limited to the compounds described in the following Examples. In addition, the manufacturing method of a raw material compound is shown in a reference example.
[0200] In addition, the following abbreviations are used in the reference example, an Example, and the table|surface mentioned later.
[0201] Ex: Example number, REx: Reference example number, Dat: Physicochemical data (F: FAB-MS (M+H) + , FN: FAB-MS (M-H) - 、ES:ESI-MS(M+H) + , EI: EI-MS(M) + 、APN: API-ES-MS(M-H) - , [The compound described as (Na) after the above-mentioned mass spectrometry measurement value indicates that it was observed as a Na salt, and the compound described as (G-2W) indicates that it was observed as a glycerol adduct dianhydrate ], NMR: DMSO-d 6 middle 1 δ (ppm) of the characteristic peak in H NMR, NMRC: CDCl 3...
reference example 1
[0203] Heating 5-bromo-2-hydroxybenzonitrile, isobutyl bromide and potassium carbonate in DMF at 80 °C in the presence of tetra-n-butylammonium bromide gives 5-bromo-2-isobutoxybenzonitrile . F: 254, 256
reference example 2
[0205] After stirring 2,2-dimethyl-1-propanol and sodium hydride in DMF at 0°C, 5-bromo-2-fluorobenzonitrile was added to react at room temperature to give 5-bromo-2-(2,2 -dimethylpropoxy)benzonitrile. NMRC: 3.67 (2H, s), 6.83 (1H, d), 7.64 (1H, d)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com