Conductive inks and manufacturing method thereof
A conductive ink, conductor technology, applied in conductive coatings, conductive layers on insulating carriers, inks, etc., can solve problems such as slow decomposition, limitation of silver compounds, lack of stability and solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
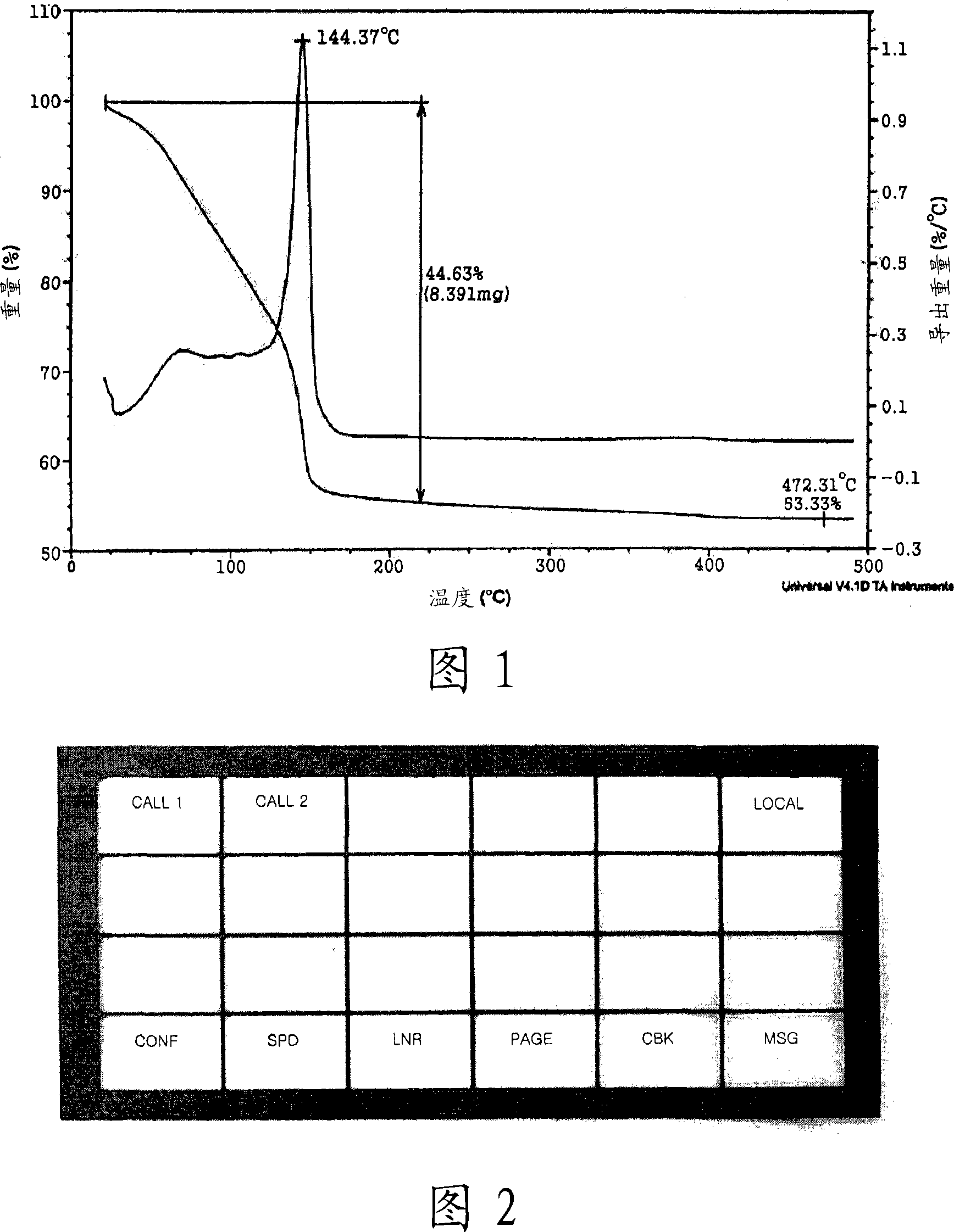
[0064] In a 50 mL Schlenk flask with a stirrer, 9.52 g (31.48 mmol) of 2-ethylhexylammonium 2-ethylhexylcarbamate (viscous liquid) was dissolved in a mixed solution containing 10.00 mL of methanol and 3.00 mL of an aqueous solution. Then, 1.00 g (15.74 mmol) of copper powder (Aldrich, particle size = 1 to 5 microns) was added and oxygen was bubbled to react for 30 minutes at room temperature. As the reaction progressed, the reaction mixture turned into a dark brown slurry and eventually a blue transparent solution. The solvent in the reaction solution was removed under vacuum to obtain 7.15 g of blue copper complex. Thermogravimetric analysis (TGA) confirmed that the copper content was 11.28% by weight. To 3.00g of the copper complex was added 5.00g copper scraps (TSC-20F, ChangSung), 0.20g binder polyvinyl butyral (BS-18, Wacker). After stirring for 10 minutes, the mixture was passed through a three-roll mill (Drais Mannheim) 5 times to obtain a conductive ink composition with a ...
Embodiment 2
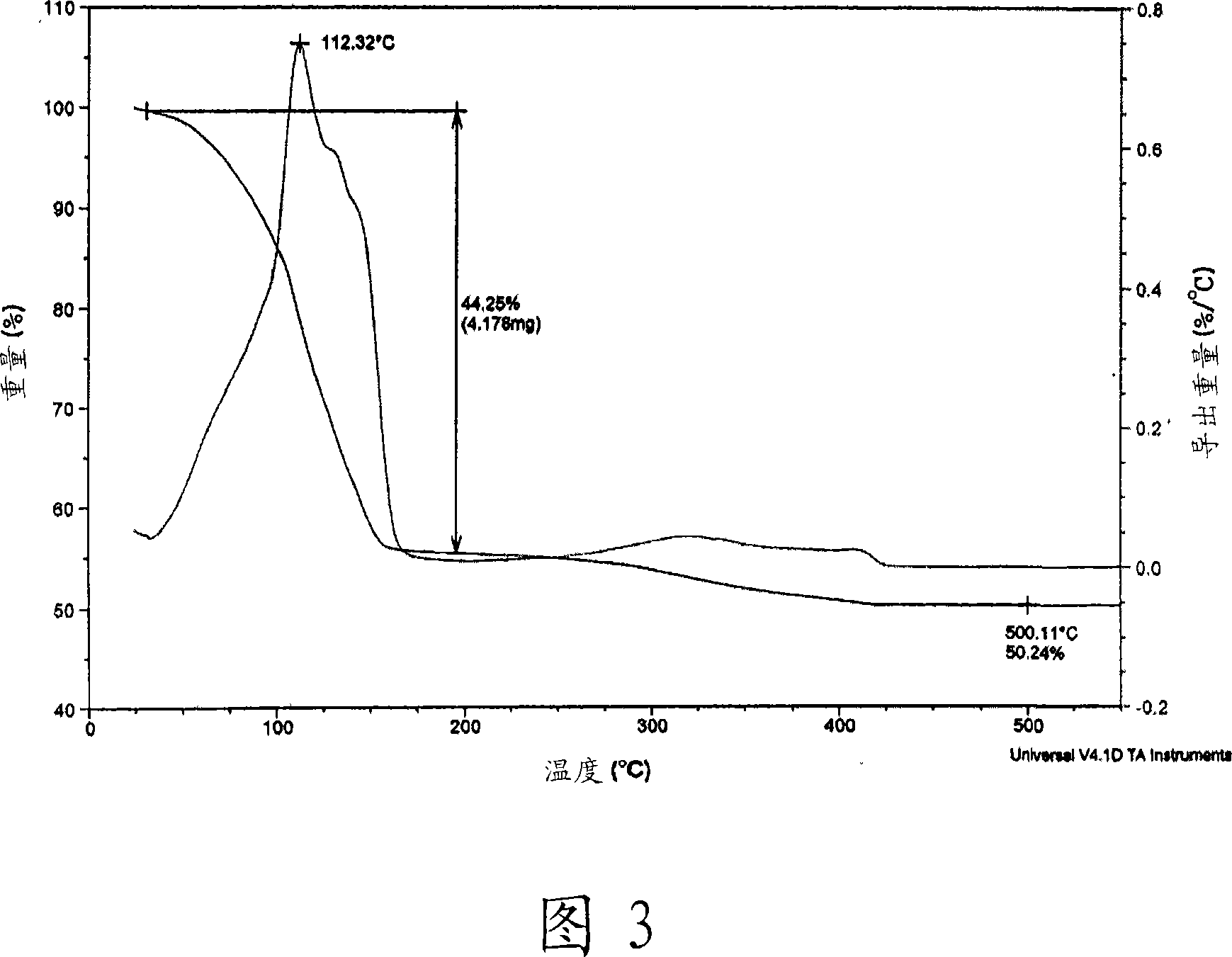
[0066] In a 50mL Schlenk flask with a stirrer, 6.99g (31.48mmol) of 3-methoxypropylammonium 3-methoxypropylcarbamate (viscous liquid) was dissolved in 2.00g containing 5.00mL methanol and 50% by weight. Hydrogen oxide (H 2 O 2 ) In a mixed solution of aqueous solutions. Then, 1.00 g (15.74 mmol) of metallic copper was added and reacted at room temperature for 2 hours. As the reaction progressed, the reaction mixture became a brown slurry and eventually a blue transparent solution. The solvent in the reaction solution was removed under vacuum to obtain 5.58 g of blue copper complex. Thermogravimetric analysis (TGA) confirmed that the copper content was 16.26% by weight. Dissolve 1.00 g of copper complex by adding 1.00 g of methanol. Then a mixture of 1:1 (molar ratio) of 2-ethylhexylammonium 2-ethylhexylcarbamate and 2-methoxyethylammonium 2-methoxyethylcarbamate was mixed with silver oxide (silver content = 22.00 (Wt%) reacted to obtain 8.00 g of the complex, thereby obtaining a t...
Embodiment 3
[0068] In a 50 mL Schlenk flask with a stirrer, 7.53 g (41.88 mmol) of di(isopropylammonium carbonate) was dissolved in 1.89 g of a mixed solution containing 20.00 mL of methanol and 50% by weight of hydrogen peroxide (H2O2) aqueous solution. Then, 1.00 g (6.98 mmol) of copper (I) oxide was added and reacted at room temperature for 2 hours. As the reaction progressed, the reaction mixture became a brown slurry and eventually a blue transparent solution. The solvent in the reaction solution was removed under vacuum to obtain 6.28 g of blue copper complex. Thermogravimetric analysis (TGA) confirmed that the copper content was 14.17% by weight. 3.00g of the copper complex was added to 2.80g of a transparent butyl cellosolve solution, and 4.00g of silver chips (EA0295, Chemet) and 0.20g of binder polyvinyl butadiene were dissolved in the butyl cellosolve solution Aldehyde (Wacker). After stirring for 10 minutes, the solution was passed through a three-roll mill 5 times to obtain a con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com