Technology for combined production of sodium carbonate and ammonium chloride through sodium sulfate type brine thermal cycle method
A technology of ammonium chloride and thermal cycle, applied in the direction of ammonium halide, carbonate preparations, etc., can solve the problems of high raw material cost, waste of resources, large discharge system, etc., and achieve strong adaptability of raw materials, high quality of main products, The effect of low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
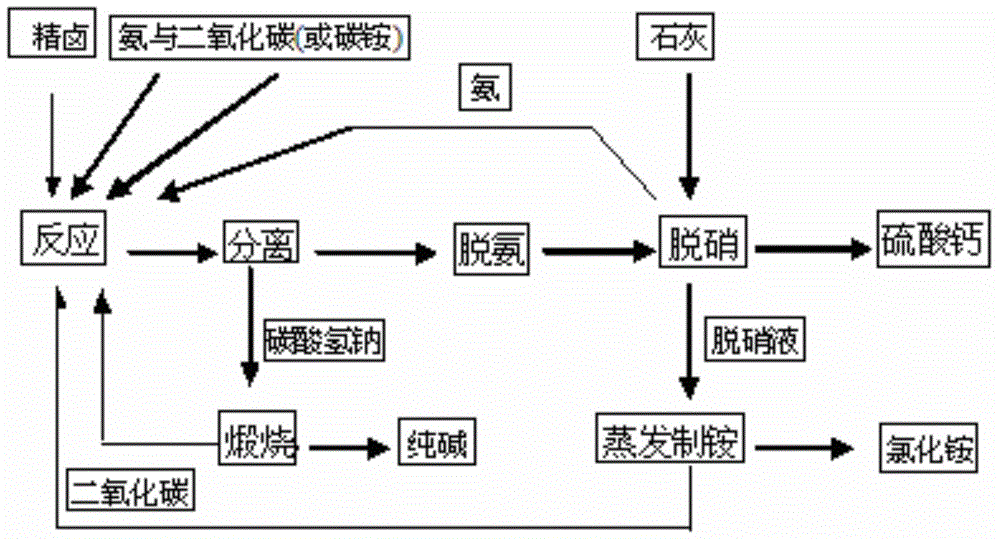
[0025] Take 100m 3 NaCl-Na 2 SO 4 -H 2 O system Glauber's salt brine (NaCl300g / L, Na 2 SO 4 10g / L) and ammonia (NH 3 ), carbon dioxide (CO 2 ) as raw material, and 50m 3 Ammonium making mother liquor (NaCl220g / L, NH 4 Cl210g / L) after metathesis carbonization reaction (reaction temperature 25 ℃), separate and obtain 43.4 tons of sodium bicarbonate (NaHCO 3 ) and 150m containing ammonium bicarbonate, ammonium chloride, sodium sulfate and sodium chloride 3 Soda making mother liquor (NaCl70g / L, NaCl 2 SO 4 6.7g / L, NH 4 Cl193.5g / L, NH 4 HCO 3 105 / L), 150m 3 Soda-making mother liquor is deaminated at high temperature (temperature 100°C) to obtain 150m 3 Deamination mother liquor (NaCl70g / L, Na 2 SO 4 6.7g / L, NH 4 Cl193.5g / L), 150m 3 Deamination mother liquor is added with nitric acid (Na 2 SO 4 6.7g / L) equivalent lime 0.52 tons (Ca(OH) 2 ) reaction to remove nitrate to obtain 0.96 tons of calcium sulfate (CaSO 4 ), ammonia (NH 3 ) and 150m 3 Denitrification ...
Embodiment 2
[0027] Take 100m 3 NaCl-Na 2 SO 4 -H 2 O system Glauber's salt brine (NaCl300g / L, Na 2 SO 4 20g / L) and ammonia (NH 3 ), carbon dioxide (CO 2 ) as raw material, and 60m 3 Ammonium making mother liquor (NaCl200g / L, NH 4 Cl270g / L) after metathesis carbonization reaction (reaction temperature 25 ℃), separate and obtain 43.4 tons of sodium bicarbonate (NaHCO 3 ) and 160m containing ammonium bicarbonate, ammonium chloride, sodium sulfate and sodium chloride 3 Soda making mother liquor (NaCl70g / L, NaCl 2 SO 412.5g / L, NH 4 Cl193.5g / L, NH 4 HCO 3 105 / L), 160m 3 Soda-making mother liquor is deaminated at high temperature (temperature 100°C) to obtain 160m 3 Deamination mother liquor (NaCl70g / L, Na 2 SO 4 12.5g / L, NH 4 Cl193.5g / L), 160m 3 Deamination mother liquor is added with nitric acid (Na 2 SO 4 12.5g / L) equivalent lime 1.04 tons (Ca(OH) 2 ) reaction to remove nitrate to obtain 1.92 tons of calcium sulfate (CaSO 4 ), ammonia (NH 3 ) and 160m 3 Denitrificatio...
Embodiment 3
[0029] Take 100m 3 NaCl-Na 2 SO 4 -H 2 O system Glauber's salt brine (NaCl300g / L, Na 2 SO 4 30g / L) and ammonia (NH 3 ), carbon dioxide (CO 2 ) as raw material, and 40m 3 Ammonium making mother liquor (NaCl240g / L, NH 4 Cl180g / L) after metathesis carbonization reaction (reaction temperature 25 ℃), separate and obtain 43.4 tons of sodium bicarbonate (NaHCO 3 ) and 140m containing ammonium bicarbonate, ammonium chloride, sodium sulfate and sodium chloride 3 Soda making mother liquor (NaCl70g / L, NaCl 2 SO 4 21.4g / L, NH 4 Cl193.5g / L, NH 4 HCO 3 105 / L), 140m 3 Soda-making mother liquor is deaminated at high temperature (temperature 100°C) to obtain 140m 3 Deamination mother liquor (NaCl70g / L, Na 2 SO 4 21.4g / LL, NH 4 Cl193.5g / L), 140m 3 Deamination mother liquor is added with nitric acid (Na 2 SO 4 21.4g / L) equivalent lime 1.56 tons (Ca(OH) 2 ) reaction to remove nitrate to obtain 2.88 tons of calcium sulfate (CaSO 4 ), ammonia (NH 3 ) and 140m 3 Denitrificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com