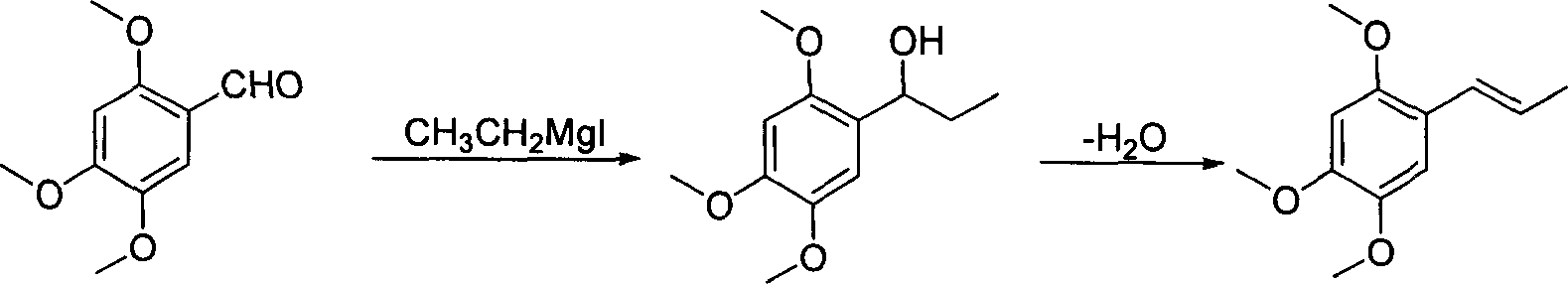
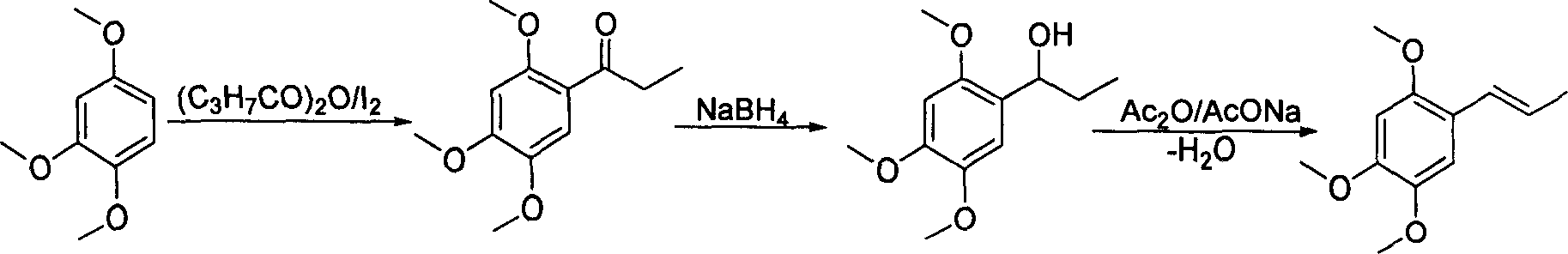
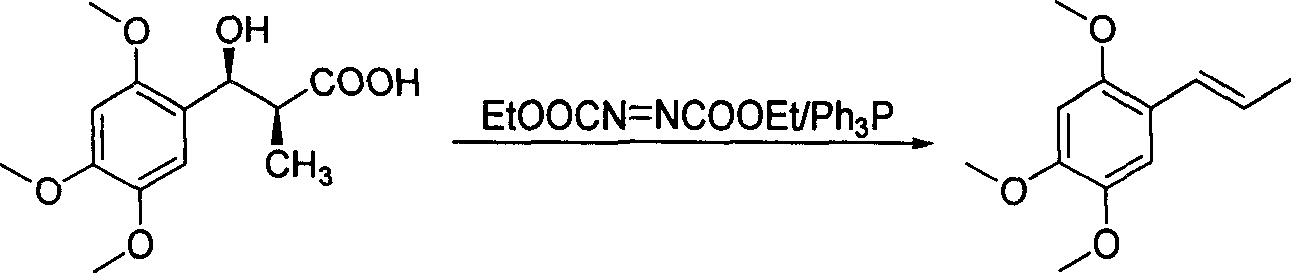Method of preparing alpha-asarone
A kind of asarone, a certain amount of technology, applied in the field of preparation of organic compounds, can solve the problems of increasing production costs, difficult to complete dehydration reaction, unsuitable for industrial production, etc., to reduce raw material costs, reduce production costs, and shorten the synthesis route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take 15g (72mmol) of mixed alkenes to a 250ml round bottom flask, add 100ml of benzene, add the catalyst PdCl 2 (CH 3 EN) 2 372 mg (1.44 mmol), heated and refluxed and stirred for 8 hours, isomerization reached equilibrium, GC showed trans / cis=93.5 / 6.5, stopped the reaction, removed the solvent under reduced pressure, added 50 ml of anhydrous diethyl ether, stirred for 5 minutes, and filtered on silica gel powder , the solvent was removed under reduced pressure to obtain an oil, which was recrystallized from n-hexane to obtain 10.7 g of white crystals, with a yield of 71.3%, and a melting point of mp60-61°C. 1 H NMR (CDCl 3 ): δ1.88 (dd, 3H, J=1.8, 6.8Hz, -CH 3 ), 3.82 (s, 3H, -OCH 3 ), 3.86(s, 3H, -OCH 3 ), 3.88(s, 3H, -OCH 3 ), 6.09(q, 1H, J=6.8, 15.7Hz, =CH), 6.49(s, 1H, PhH), 6.65(d, 1H, -CH=, 15.7Hz), 6.94(s, 1H, PhH) .
Embodiment 2
[0036] Take 5g (24mmol) of mixed alkenes to a 100ml round bottom flask, add 25ml CH 2 Cl 2 , adding catalyst PdCl 2 (PhCN) 2 184mg (0.48mmol), stirred at room temperature for 24h, isomerization reached equilibrium, GC showed trans / cis=94.0 / 6.0, stopped the reaction, removed the solvent under reduced pressure, added 25ml of anhydrous ether, stirred for 5min, and filtered on silica gel powder , the solvent was removed under reduced pressure to obtain an oil, which was recrystallized from n-hexane to obtain 3.1 g of white crystals, with a yield of 62.0%, melting point mp60-61°C, 1 H NMR (CDCl 3 ): δ1.88 (dd, 3H, J=1.8, 6.8Hz, -CH 3 ), 3.82 (s, 3H, -OCH 3 ), 3.86(s, 3H, -OCH 3 ), 3.88(s, 3H, -OCH 3 ), 6.09(q, 1H, J=6.8, 15.7Hz, =CH), 6.49(s, 1H, PhH), 6.65(d, 1H, -CH=, 15.7Hz), 6.94(s, 1H, PhH) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com