Fluorescent probe for detecting ultra-oxygen anion free radical, synthesis method and use
A technology of superoxide anion and fluorescent probe, which is applied in the field of biological detection technology and clinical medical detection, can solve the problems of short free radical life, difficult detection, and many types, and achieve stable performance, no toxic and side effects, and small toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
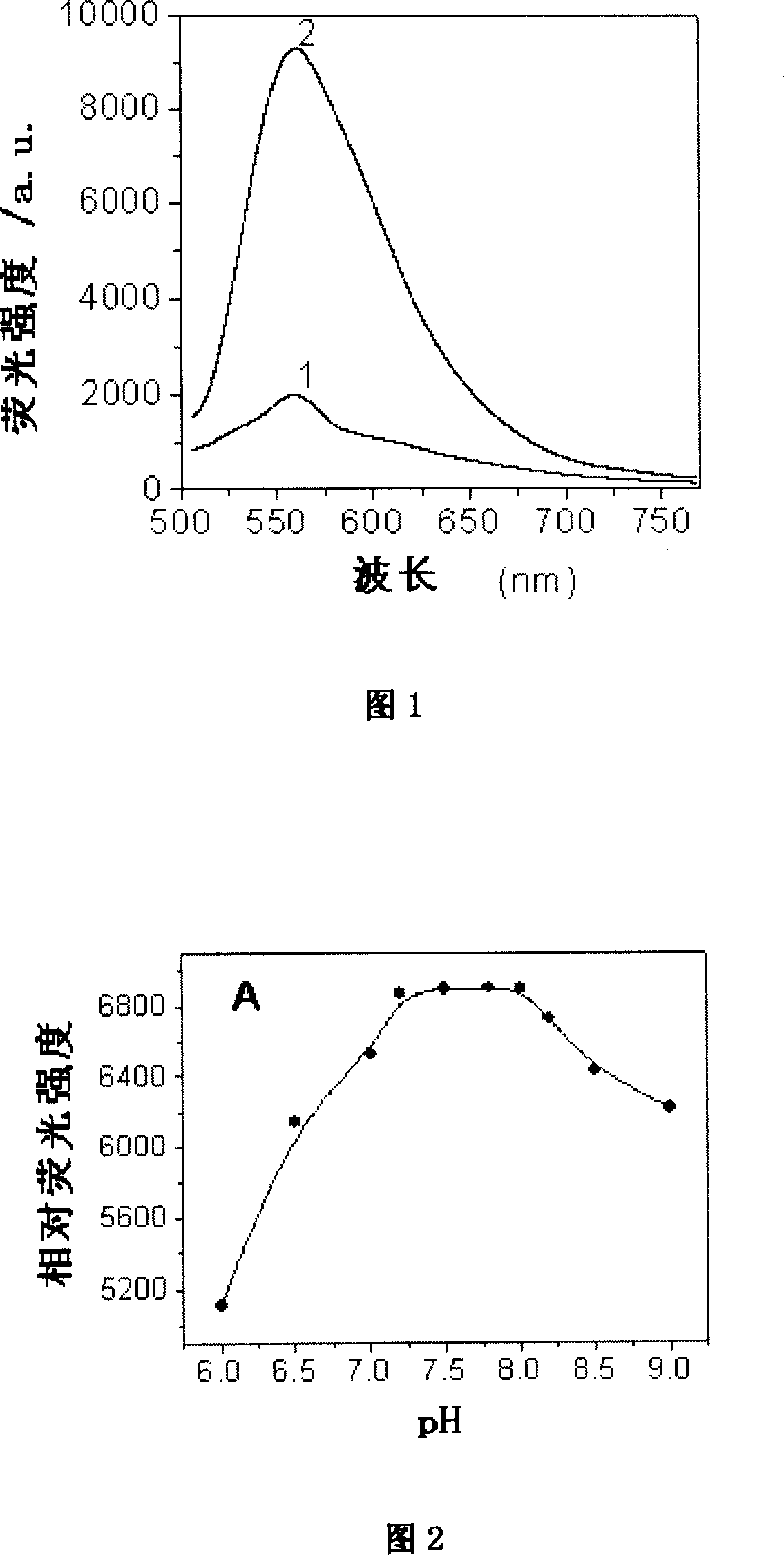
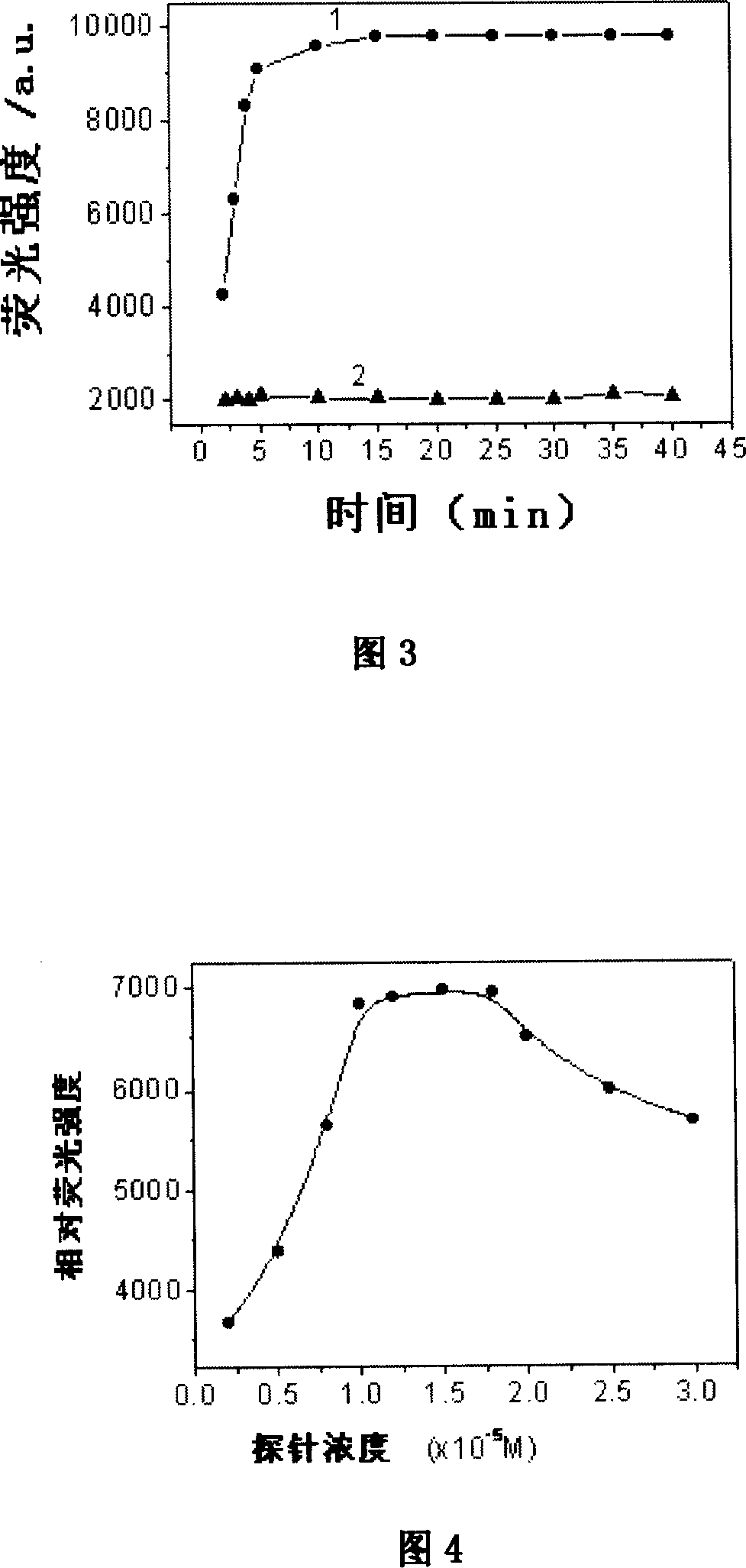
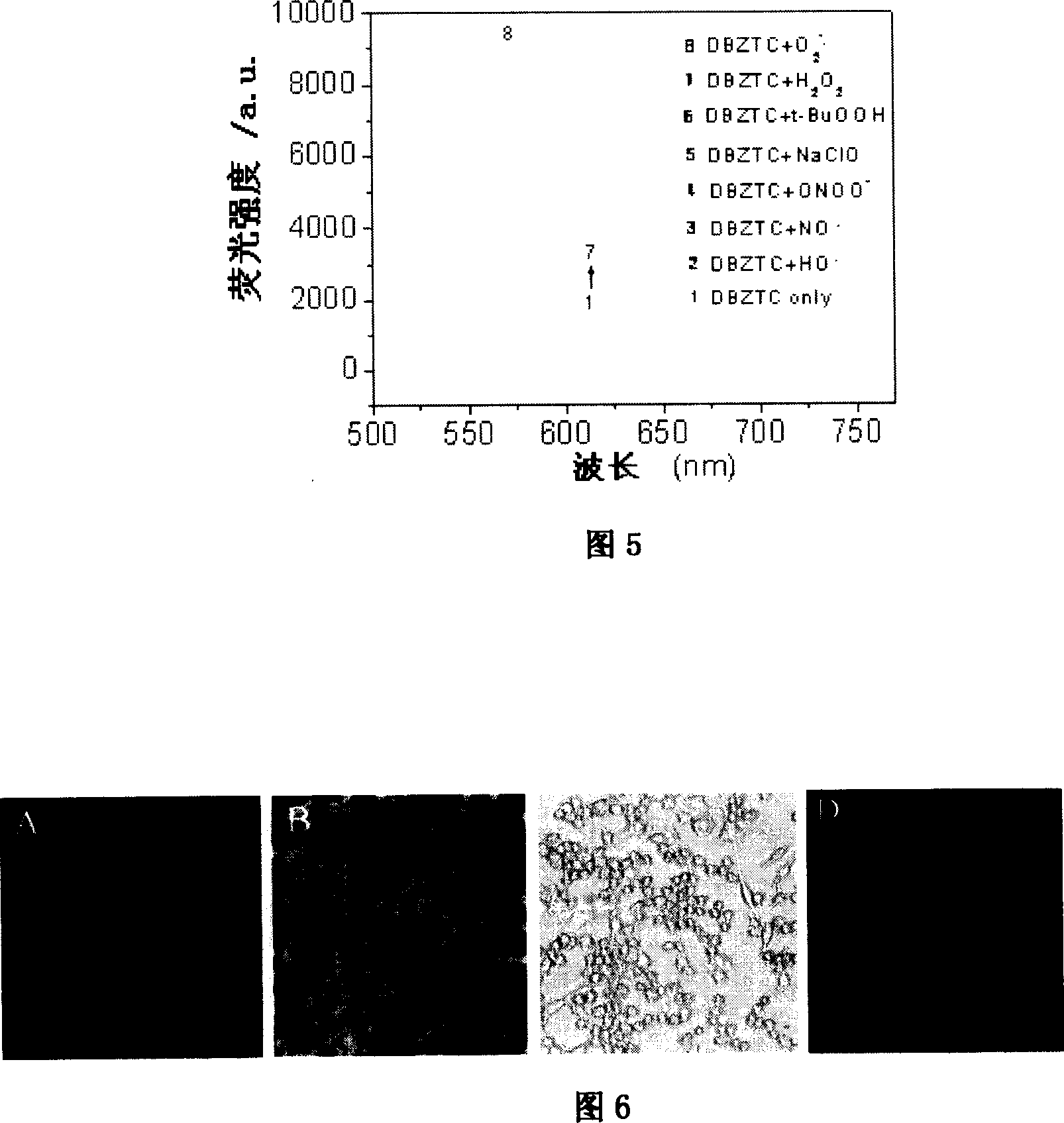
Image
Examples
Embodiment 1
[0043] Probe Synthesis
[0044]
[0045] Probe DBZTC
[0046] Get 17 grams of chlorinated dialdehyde-based cyclohexene and 35 grams of o-aminothiophenol and dissolve in the mixed solution of 500 milliliters of n-butanol and 300 milliliters of benzene to obtain a mixed solution; the mixed solution was refluxed for 3 hours, and the Remove the solvent under pressure (0.02 atmospheres, 55°C) to obtain a crude purple-red fluorescent probe; then recrystallize the crude product with 40 ml of ethanol with a weight concentration of 99%, and vacuum-dry (0.01 atmospheres) to obtain a brown-purple fluorescent probe Pure product (54% yield).
[0047] 1 H NMR (300MHz, DMSO-d 6 , 25°C, TMS): 8.10-7.90(m, 2H), 7.75(d, 2H), 7.47-7.39(m, 2H), 7, 24(d, 2H), 4.10(m, 2H), 3.12(m, 2H), 2.3( m, 3H), 1.15 (m, 4H).IR (KBr tablet): v (cm -1 ) 3245 (N-H), 3110 (C-H), 1592 (C=C). Elemental analysis results (theoretical value): C 20 h 19 N 2 S 2 Cl: C 62.10 (62.02), H 4.92 (4.84), N 7.24 (7....
Embodiment 2
[0049] Probe Synthesis
[0050]
[0051] Get 17 grams of chlorinated dialdehyde cyclohexene and 59 grams of methyl o-aminothiophenol and dissolve them in a mixed solution of 500 milliliters of n-butanol and 150 milliliters of benzene to obtain a mixed solution; the mixed solution was heated to reflux for 3 hours , the solvent was evaporated under reduced pressure (0.01 atmosphere, 45° C.) to obtain the crude product of the purple-red fluorescent probe; Pure probe (yield 54.8%).
Embodiment 3
[0053] Probe Synthesis
[0054]
[0055] Get 17 grams of chlorinated dialdehyde cyclohexene and 48 grams of ethyl o-aminothiophenol and dissolve them in a mixed solution of 500 milliliters of n-butanol and 220 milliliters of benzene to obtain a mixed solution; the mixed solution was heated to reflux for 4 hours , the solvent was evaporated under reduced pressure (0.01 atmosphere, 50° C.) to obtain the crude product of the purple-red fluorescent probe; Pure probe (yield 54.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com