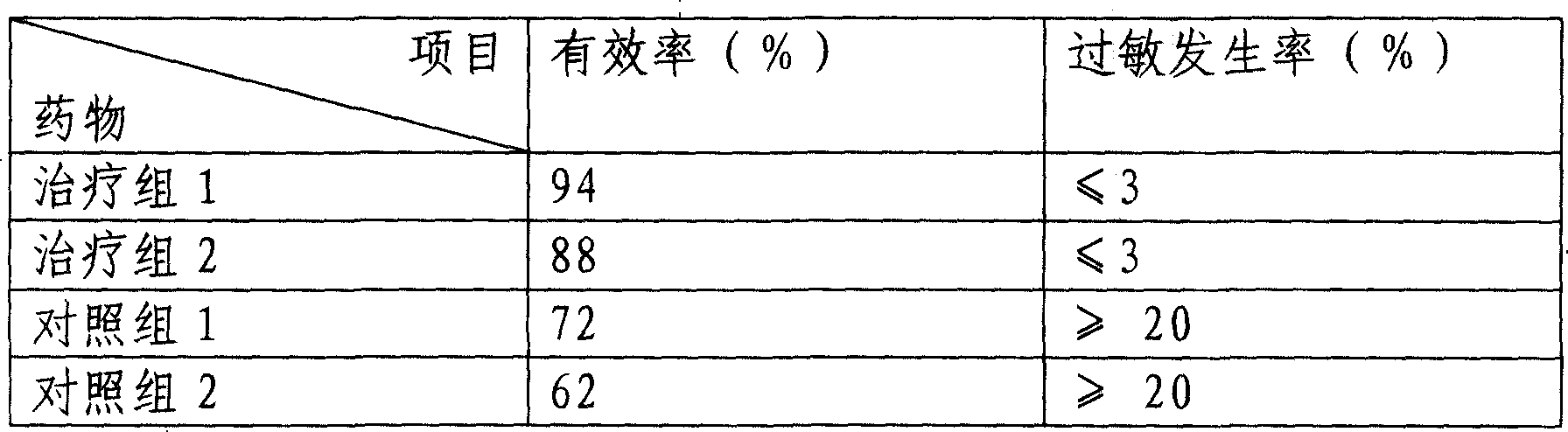
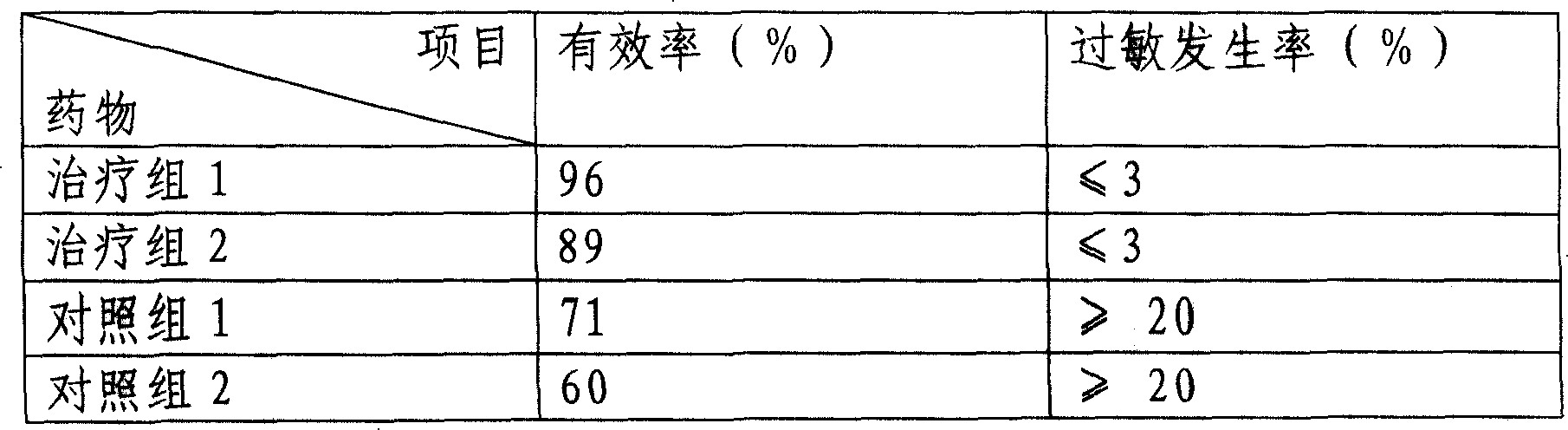
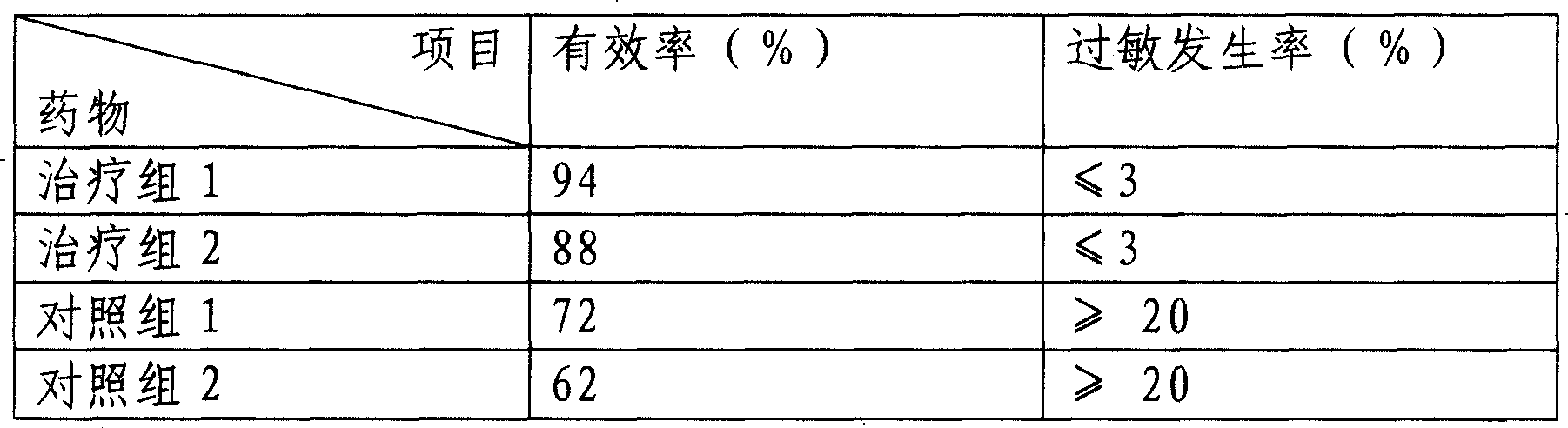
Nanometer cytochrome liposome medicine and its prepn
A technology of nano-liposome and cytochrome, which is applied in the direction of liposome delivery, drug combination, pharmaceutical formulation, etc., can solve the problems of unstable curative effect, existence of allergies, impact on application, etc., and achieve reduction of allergies and residues Small, increased targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In the formula adopted in the present embodiment, each raw material and parts by weight thereof are as follows:
[0037] Neutral phospholipid 40;
[0038] Sitosterol 20;
[0039] Cholesterol 20;
[0040] Cytochrome C 30;
[0041] Polyethylene glycol 20;
[0042] Reduced Glutathione 10;
[0043] Vitamin C 40.
[0044] The neutral phospholipid refers to a mixture of hydrogenated soybean lecithin and cephalin in a weight ratio of 1-3:1, the sitosterol refers to β-sitosterol, and the polyethylene glycol refers to polyethylene glycol 4000.
[0045] Hydrogenated soybean lecithin is mixed with cephalin, sitosterol, and cholesterol to form the special liposome structure of the drug of the present invention. Polyethylene glycol is incorporated into liposomes to form long-circulating liposomes. Glutathione is the Antioxidant and in vivo activator of cytochrome C, vitamin C is excipient and in vitro antioxidant.
[0046] The preparation steps are as follows:
[0047] (1) D...
Embodiment 2
[0059] In the formula adopted in the present embodiment, each raw material and parts by weight thereof are as follows:
[0060] Neutral phospholipid 80;
[0061] Sitosterol 10;
[0062] Cholesterol 40;
[0063] Cytochrome C 10;
[0064] Polyethylene glycol 25;
[0065] Reduced Glutathione 5;
[0066] Vitamin C 50.
[0067] The neutral phospholipid refers to a mixture of hydrogenated soybean lecithin and cephalin in a weight ratio of 1-3:1, the sitosterol refers to β-sitosterol, and the polyethylene glycol refers to polyethylene glycol 4000.
[0068] Hydrogenated soybean lecithin is mixed with cephalin, sitosterol, and cholesterol to form the special liposome structure of the drug of the present invention. Polyethylene glycol is incorporated into liposomes to form long-circulating liposomes. Glutathione is the Antioxidant and in vivo activator of cytochrome C, vitamin C is excipient and in vitro antioxidant.
[0069] The preparation steps are as follows:
[0070] (1) Diss...
Embodiment 3
[0082] In the formula adopted in the present embodiment, each raw material and parts by weight thereof are as follows:
[0083] Neutral phospholipid 80;
[0084] Sitosterol 20;
[0085] Cholesterol 40;
[0086] Cytochrome C 30;
[0087] Polyethylene glycol 25;
[0088] Reduced Glutathione 10;
[0089] Vitamin C 50.
[0090] The neutral phospholipid refers to a mixture of hydrogenated soybean lecithin and cephalin in a weight ratio of 1-3:1, the sitosterol refers to β-sitosterol, and the polyethylene glycol refers to polyethylene glycol 4000.
[0091] Hydrogenated soybean lecithin is mixed with cephalin, sitosterol, and cholesterol to form the special liposome structure of the drug of the present invention. Polyethylene glycol is incorporated into liposomes to form long-circulating liposomes. Glutathione is the Antioxidant and in vivo activator of cytochrome C, vitamin C is excipient and in vitro antioxidant.
[0092] The preparation steps are as follows:
[0093] (1) D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com