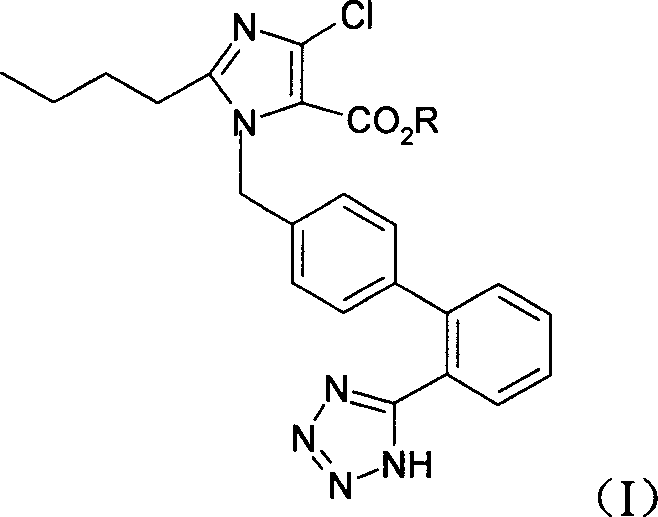
Imidazo-5-carboxylic-acid derivatives, its preparing method and use
A kind of imidazole and compound technology, applied in imidazole-5-carboxylic acid derivatives, preparation and application fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 2-Butyl-4-chloro-1-[2`-(1H-tetrazol-5-yl)1,1`-biphenyl-methyl]imidazole-5-carboxylic acid
[0085]
[0086] Add 10ml water to 2-butyl-4-chloro-1-[2`-(1H-tetrazol-5-yl)1,1`-biphenyl-methyl]-5-hydroxymethylimidazole 4.57g Dissolve, cool to -5-0°C, add 1.58g KMnO dropwise 4 130ml of aqueous solution, after dropping, react at 50°C for 16h. Stop the reaction, filter, add 1mol / L NaS to the filtrate 2 o 3 50ml, then adjust the pH of the solution to 2-3 with dilute hydrochloric acid, the solution becomes cloudy, extract with ethyl acetate, dry, concentrate, pass through a flash column, and use petroleum ether: ethyl acetate = 1:6 as the mobile phase to obtain a white solid 3.85g, yield 89.1%.
[0087] 1HNMR (CDC13):
[0088] δH 0.801(3H, t, J=3.6), 25(2H, m, J=3.5), 1.49(2H, m, J=5), 2.56(2H, t, J=3.5), 5.58(2H, s ), 6.94-7.08 (4H, m, J=5), 7.65-7.50 (2H, m, J=8.5)
[0089] ESI(-): 435.1
[0090] MP: 125.2-128.5°C
Embodiment 2
[0092] 2-Butyl-4-chloro-1-[2`-(1-trityl-tetrazol-5-yl)1,1`-biphenyl-methyl]imidazole-5-carboxylic acid
[0093]
[0094] In a 100ml one-port bottle, add 2-butyl-4-chloro-1-[2`-(1H-tetrazol-5-yl)1,1`-biphenyl-methyl]imidazole-5-carboxyl Acid 4.36g, 15ml N,N-dimethylformamide, 1.66g potassium carbonate and 2.78g triphenylchloromethane, react overnight at room temperature, stop the reaction, add 100ml water, extract with 100ml ethyl acetate, and wash with saturated brine Once, the organic phase was dried and concentrated to give yellow oil 2-butyl-4-chloro-1-[2`-(1-trityl-tetrazol-5-yl)1,1`-biphenyl -Methyl]imidazole-5-carboxylic acid 7.5 g.
[0095] The crude product obtained in this example was used as the raw material mentioned in the following examples without purification.
Embodiment 3
[0097] 2-Butyl-4-chloro-1-[2`-(1H-tetrazol-5-yl)1,1`-biphenyl-methyl]imidazole-5-carboxylic acid ethyl ester
[0098]
[0099] Add 15ml of absolute ethanol and 312mg of p-toluenesulfonic acid (TsOH) to 678.5mg of raw materials, and reflux for 6h. After the reaction, add 30ml of water, extract with 30ml of ether, dry the organic phase, and concentrate to obtain the colorless oily product 2-butyl-4 -Ethyl chloro-1-[2'-(1H-tetrazol-5-yl)1,1'-biphenyl-methyl]imidazole-5-carboxylate 274 mg, yield 59%.
[0100] 1 H-NMR (CDCl 3 )
[0101] δH(ppm): 0.80-0.85(m, 6H, J=13.6), 1.26(m, 2H, J=20.2), 1.38(H, t, J=14.8), 1.58(m, 2H, J=7.5) , 2.69(q, 2H, J=24.5), 5.44(s, 2H), 6.94-7.50(8H), 8.10(d, 1H, J=6.14)
[0102] ESI (+) m / z: 465.1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com