Sustained local anesthetic composition containing preferably a sugar ester such as saib
A technology of local anesthesia and local anesthetics, applied in the field of controlled release systems, which can solve problems such as difficult to overcome and unavoidable systemic initial burst release of anesthetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] The non-polymeric liquid carrier system containing the anesthetic was fabricated as follows. Sucrose acetate isobutyrate (SAIB) was mixed with N-methylpyrrolidone (NMP), a solvent for the SAIB vehicle, to form a 70:30 mixture. To this mixture was added either 2.5% (w / v (weight / volume)) or 5% bupivacaine (free base) to provide two test compositions.
[0165] Male Sprague Dawley rats (275g-300g) were divided into two test groups of 8 animals each. The test formulation was injected into the quadruped using a needle and cannula at either 25 mg or 50 mg bupivacaine doses. The presence of local anesthesia was then determined using a skin contraction response test in which there was an instinctive contraction of the skin stimulated by needle sticks in 10 random areas within 1 cm of the injection site. The percentage of needle sensory inhibition was calculated by subtracting the test response from the baseline response and dividing by the baseline response and multiplying by ...
Embodiment 2
[0168] subject. Male Fisher 344 rats (Charles River Laboratories) (N=96) were used for the study. Animals were maintained on a reversed light-dark cycle (dark from 5:00 to 17:00) in a temperature-humidity-controlled vivarium. Rats were given ad libitum (except during the experimental period) access to food and water. All tests were performed during the dark phase of the light-dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest University Health Science Center.
[0169] surgical procedure. After induction of anesthesia with 5% isoflurane vapor in oxygen, the left lower abdominal region of the animals was shaved. Anesthesia was maintained during the operation using 2.0% to 2.5% isoflurane vapor in oxygen. A 3 cm incision was made at a position 0.5 cm below the lowermost rib on the left side and parallel to the rib, and the incision penetrated into the abdominal cavity. Viscera and musculature are strongly stimulated by...
Embodiment 3
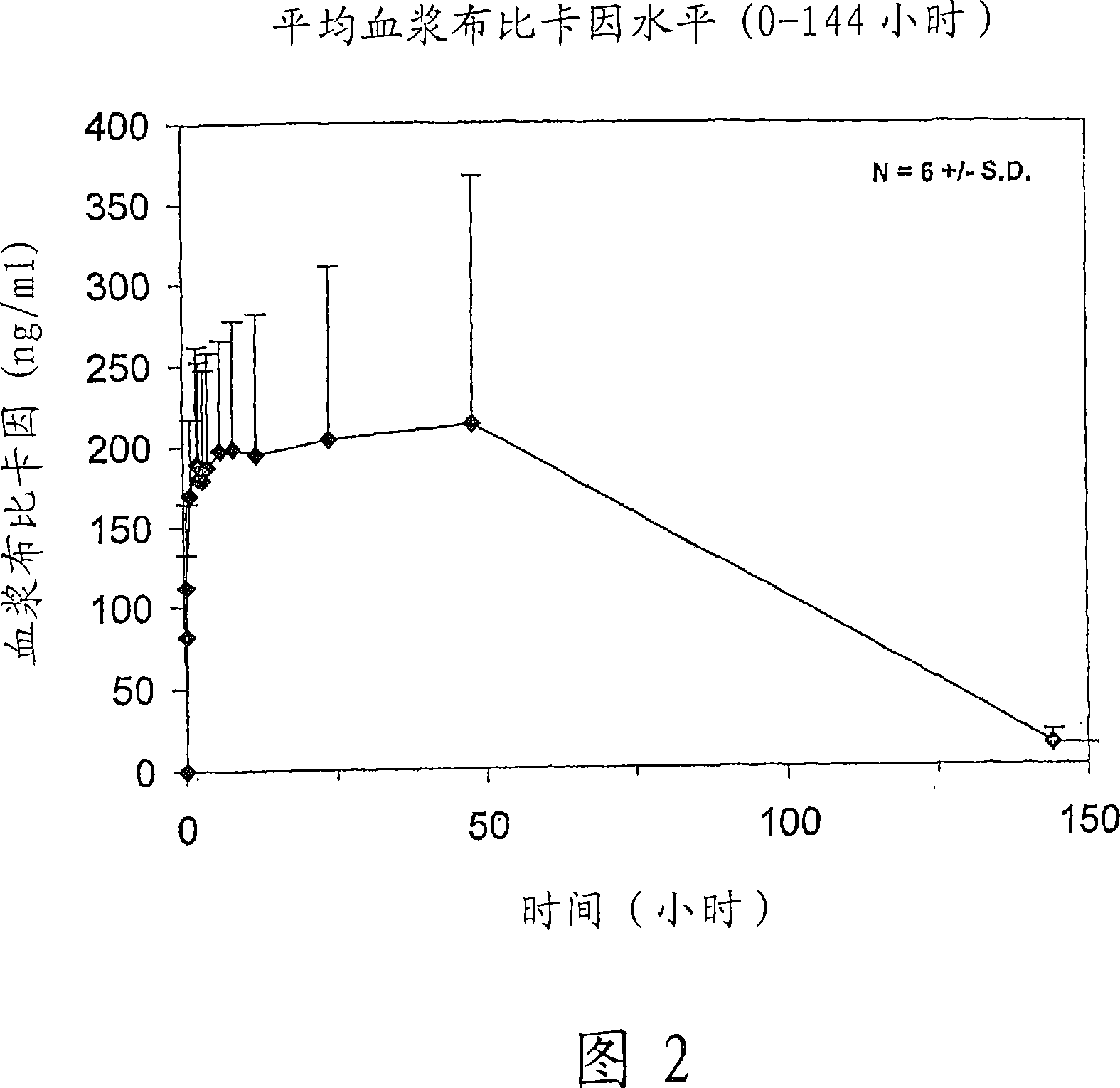
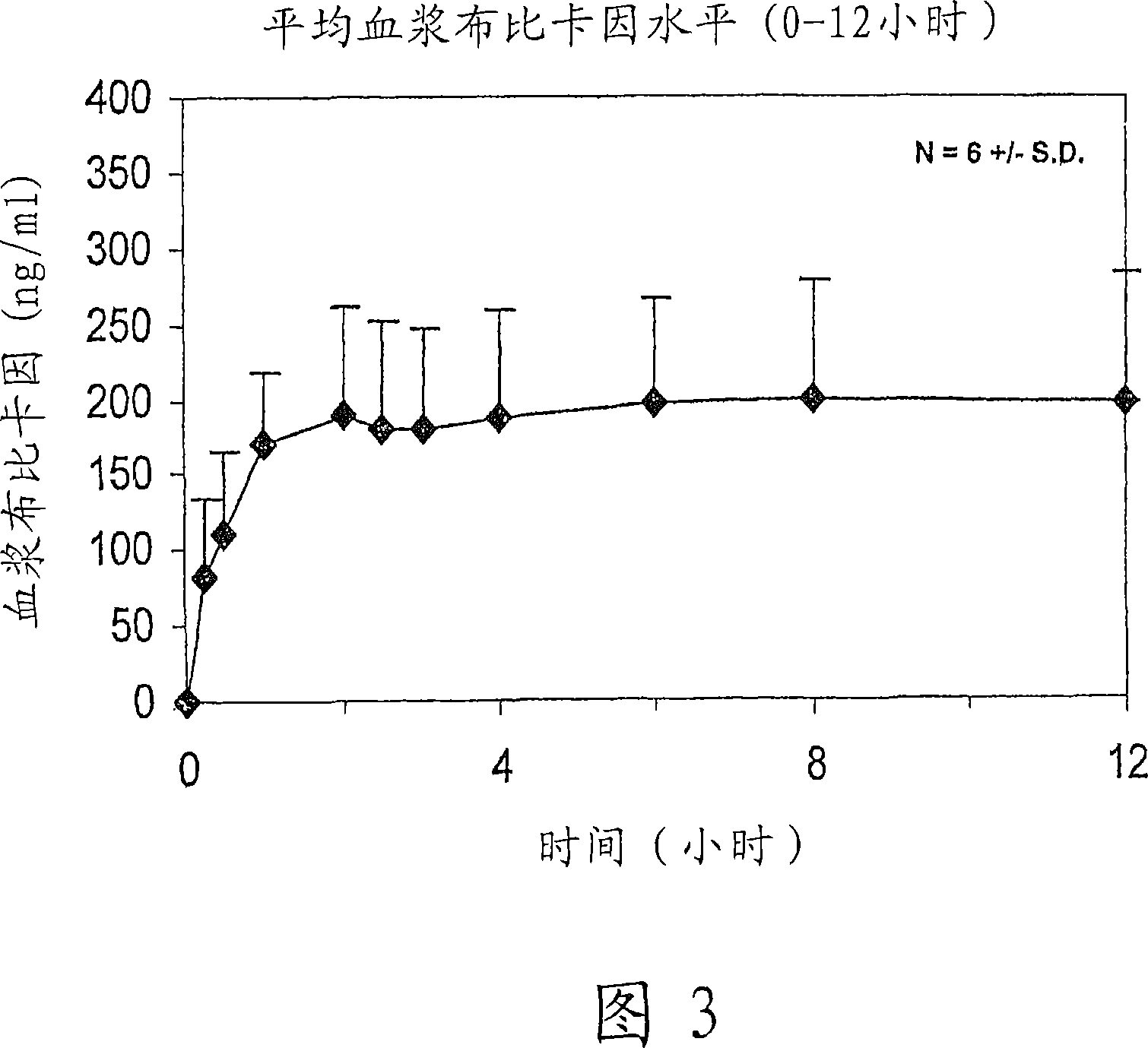
[0216] The following dose escalation, safety and pharmacokinetic evaluations were performed in healthy human volunteer subjects to evaluate the safety / tolerance of controlled release bupivacaine compositions containing sucrose acetate isobutyrate non-polymeric carriers and preliminary pharmacokinetic properties.
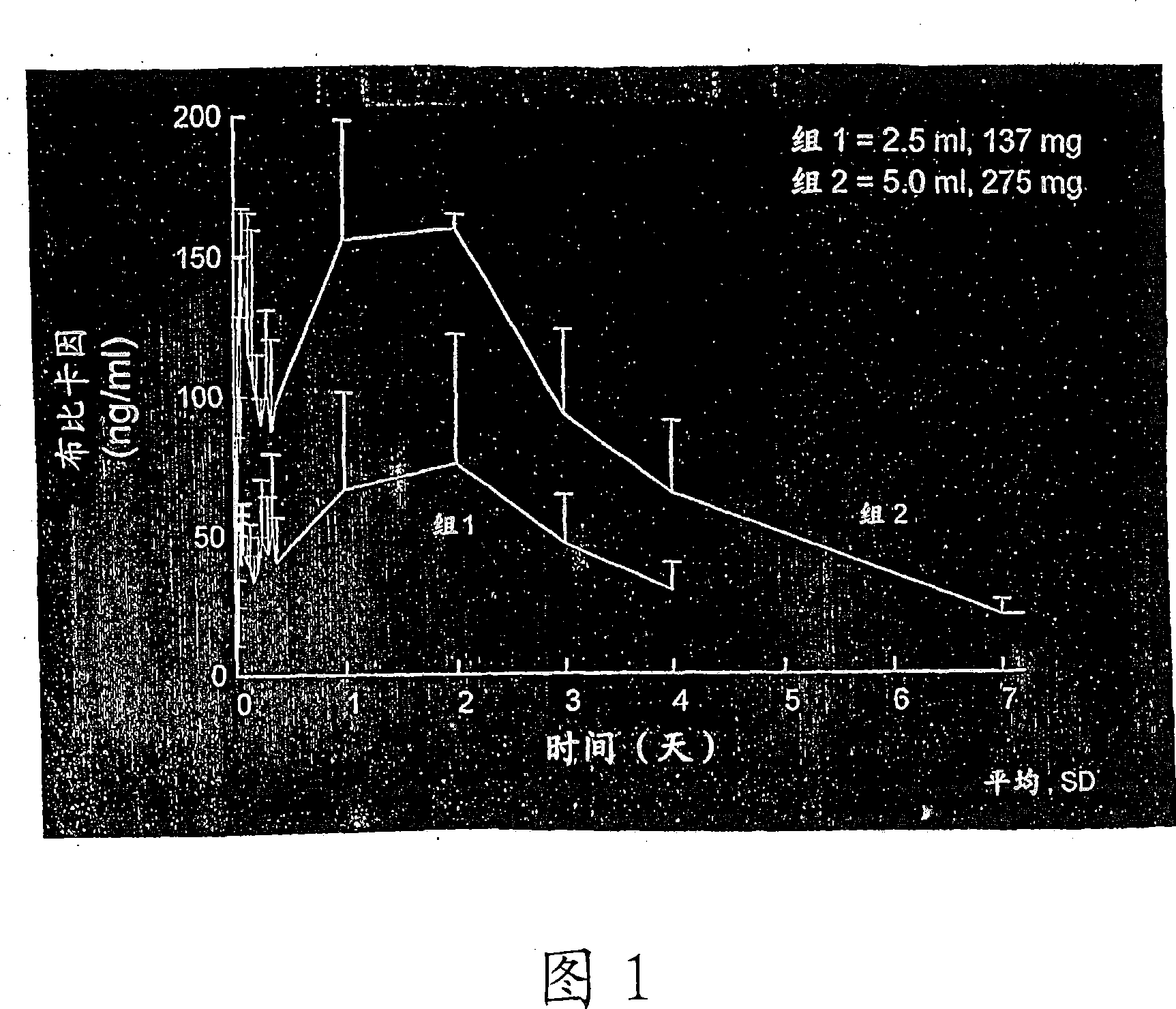
[0217] Bupivacaine formulated in a sucrose acetate isobutyrate (SAIB) non-polymeric vehicle further comprising N-methyl-2-pyrrolidone (NMP) used as a solvent for the bupivacaine and SAIB vehicle was used The composition was prepared from the free base. The composition was prepared by combining the SAIB vehicle and NMP solvent (70:30 vehicle substance) with 5% by weight bupivacaine to provide an individual containing 137.5 mg bupivacaine in a 2.5 mL injection volume dose. The composition is provided as an injectable solution.
[0218]There were two groups in the study. Group 1 included 6 healthy male subjects, aged 22-38 years. For Group 1, all subjects received ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap