Gene engineering bacterium of beta - glucosaccharase, and application
A technology of genetically engineered bacteria and glucosidase, which is applied in the application field of preparing effective mycotoxamine A compounds, can solve the problem of being difficult to prepare and separate effective mycotoxamine A and the like, and achieve the target product effective mycotoxane A. The effect of large amount of base amine A, stable and single product, mild and easily controllable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
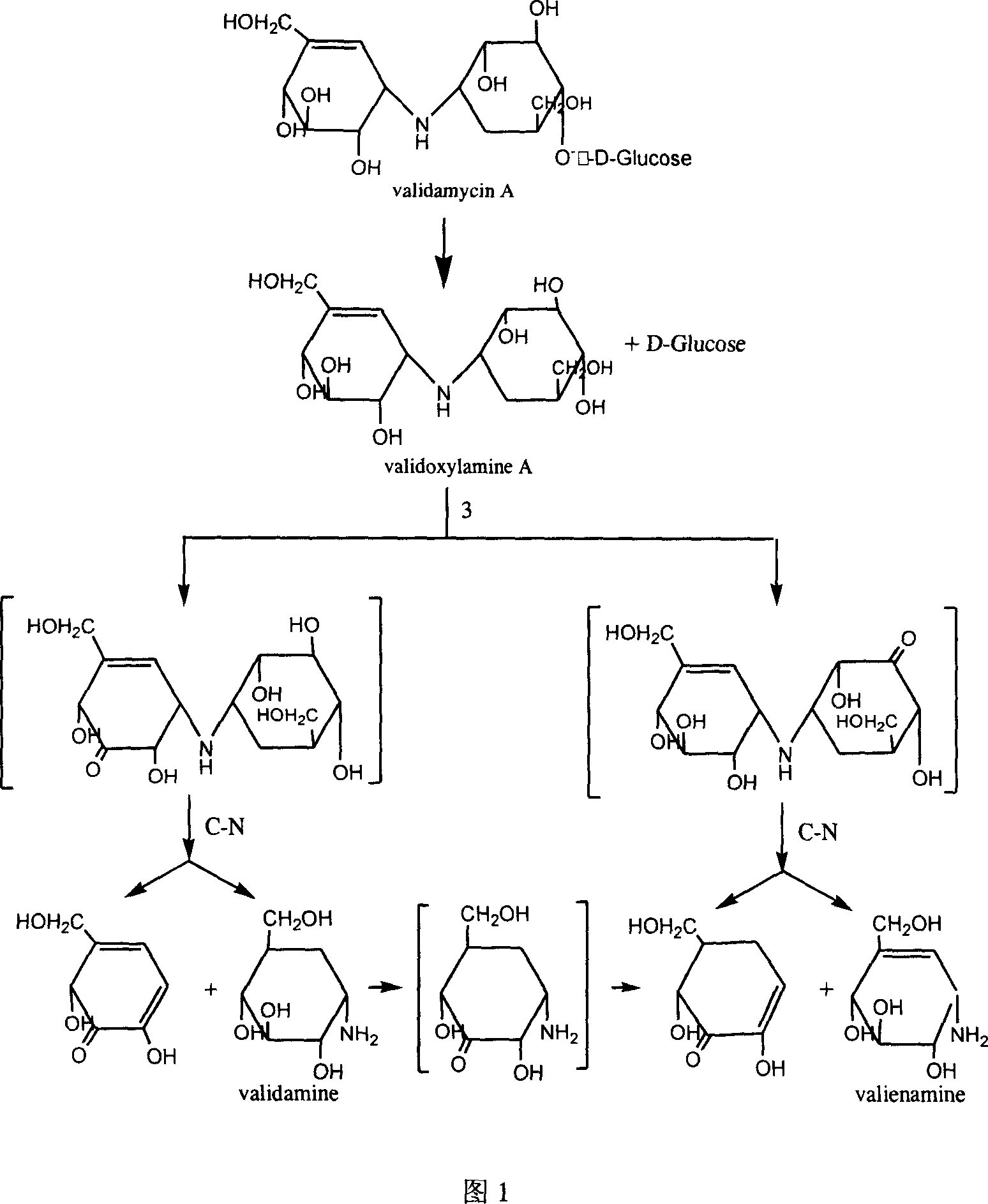
[0016] Construction of engineering bacteria CGMCC No.1626
[0017] Specific PCR amplification primers were designed and synthesized according to a β-glucosidase gene sequence in the reported complete gene sequence of Agrobacterium tumefaciens str.C58 (GenBank accession no.NC_003063). The 5' ends of the two primers contained a single HindIII. and SacI cleavage site.
[0018] P1: 5'-GC AAGCTT TCACCCCTTCACCACACC-3' (the underlined HindIII restriction site), P2: 5'GCGG GAGCTC ATGGATAGAAAGGCCTCT-3' (SacI restriction site is underlined). Using the genome of Agrobacterium sp.HCCB-20052 as a template, the β-glucosidase gene of primers P1 and P2 was used for PCR amplification. The PCR reaction system was 50 μl, containing 5 ng of Agrobacterium sp. HCCB-20052 total DNA, 25 μl of Premix Taq, and 50 pmol of each primer. Reaction conditions: denaturation at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for...
Embodiment 2
[0021] Cultivation of engineered bacteria
[0022] The recombinant engineered bacteria were inoculated into LB medium containing kanamycin (50 μg / ml) and cultured with shaking at 37°C to logarithmic growth phase (OD). 600 to about 0.6), add 1 mM IPTG or lactose, and induce culture at 37°C for 4 hours.
Embodiment 3
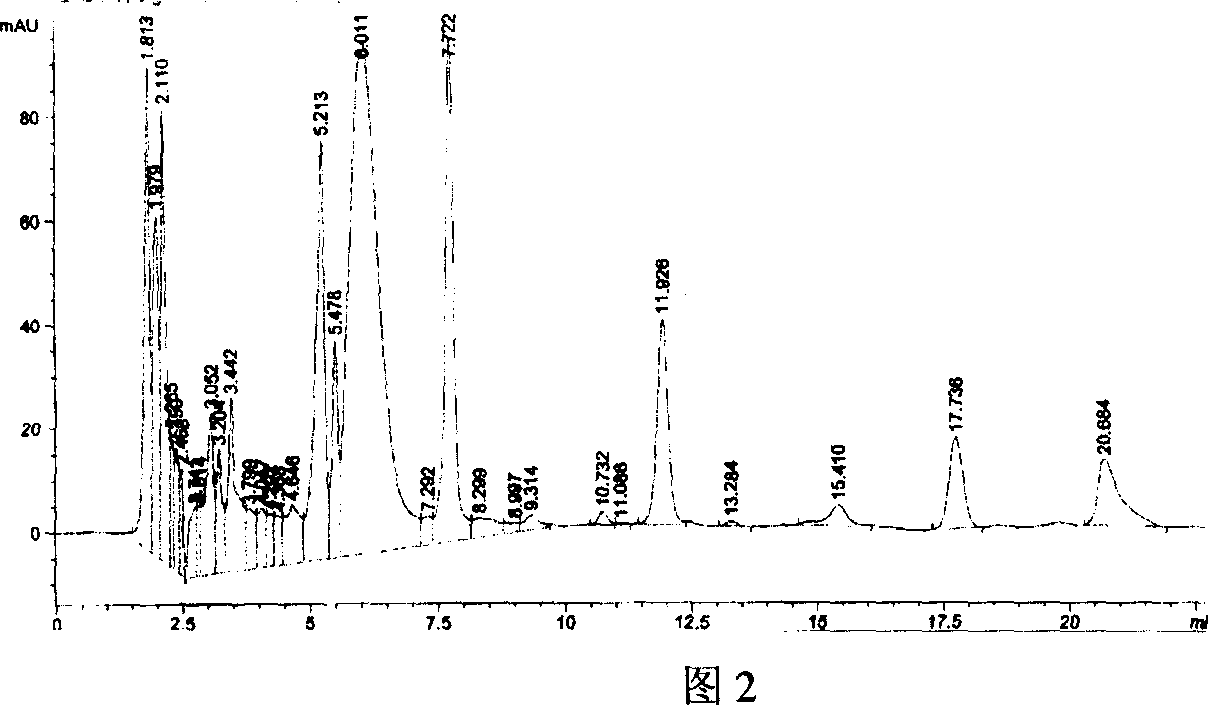
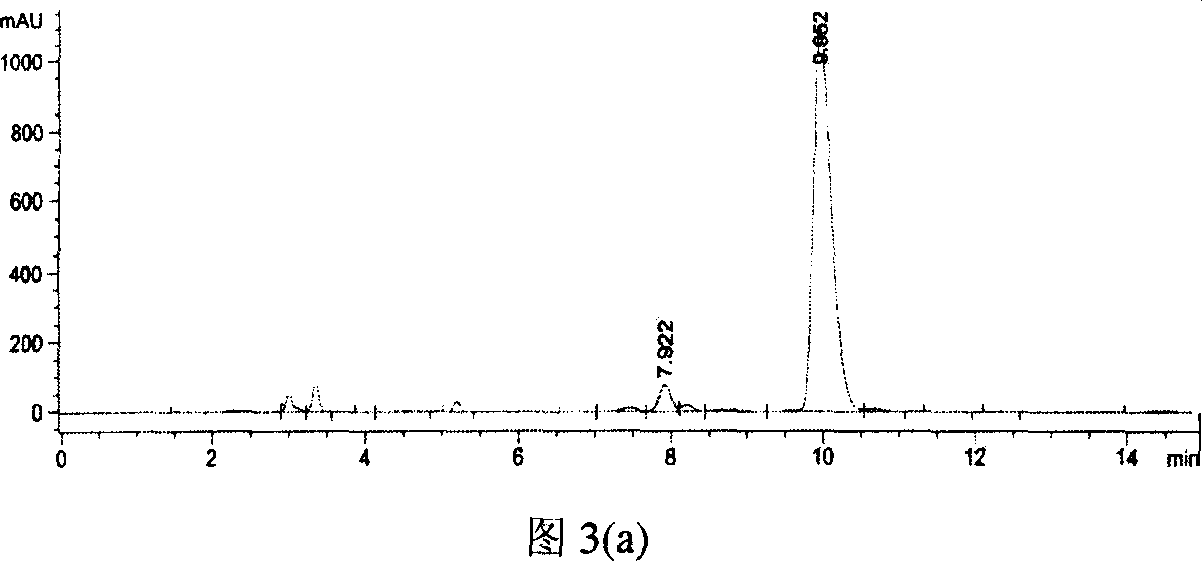
[0024] Obtaining of Crude Enzyme and Conversion of Crude Enzyme to Effective Validamycin A
[0025] The induced cultured recombinant bacteria were centrifuged at 4000rpm for 15min at room temperature to collect the bacteria, washed three times with PBS buffer, and then used 1 / 10 volume of 0.05M, pH5.5 citric acid / sodium citrate buffer The bacteria were resuspended, sonicated, and incubated at 4°C with Centrifuge for 25 min and take the supernatant as crude enzyme.
[0026] The crude enzyme solution was diluted 20 times, 20 ml was taken out, 0.1 g of effective valomycin A substrate was added, and the conversion reaction was carried out at 32°C. 54% conversion after 1 hour of conversion
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com