External applied preparations for treating intractable skin ulcer
A technology for skin ulcers and external preparations, applied in the direction of medical preparations containing active ingredients, skin diseases, organic active ingredients, etc., can solve the problems of limited clinical application range and unsatisfactory curative effect, and achieve prevention and control of infection and exudation Less, strong astringent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
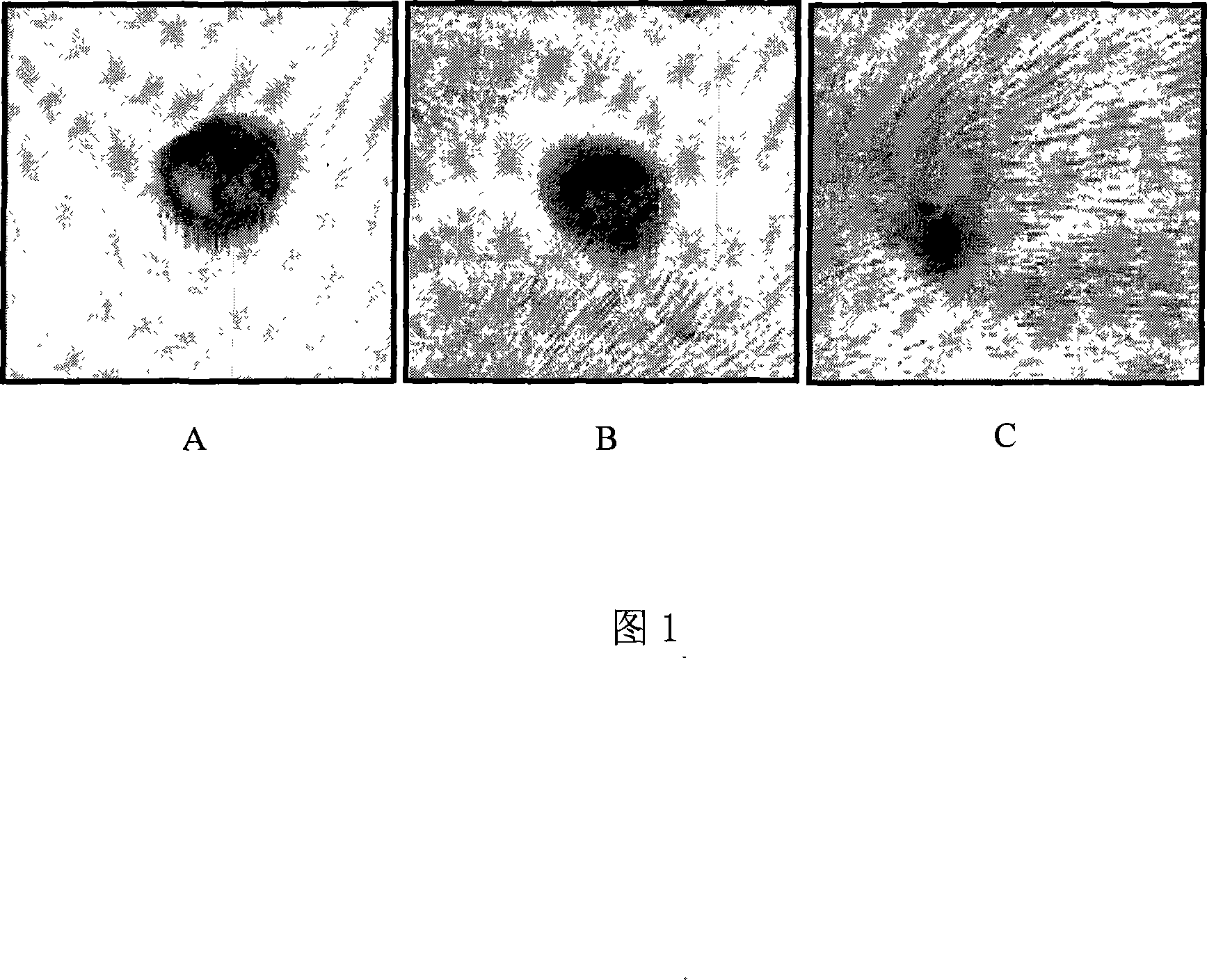
Image
Examples
Embodiment 1
[0032] Example 1 consists of the active ingredients of the formulation by the following weights:
[0033] Insulin (human insulin) (5000u)
[0034] Epidermal Growth Factor (5mg)
[0035] Silver Sulfadiazine (10g)
[0036] Triethanolamine (20g)
[0037] Stearic acid (100g)
[0038] Glycerin (50g)
[0039] Lanolin (20g)
[0040] White Vaseline (150g)
[0041] Ethylparaben (1g)
[0042] Add distilled water to 1000g
[0043] Preparation method: the oil phase is stearic acid, lanolin, white petrolatum and ethyl paraben; the water phase is triethanolamine, glycerin and water; the water phase and the oil phase are stirred and heated to 70-80 ℃ respectively, and then the oil phase Slowly add the water phase while stirring well; when the temperature drops below 40°C, add insulin, epidermal growth factor and silver sulfadiazine, stir well and grind evenly, and t...
Embodiment 2
[0045] Example 2 consists of the following weights of formulation active ingredients:
[0046] Insulin (Bovine Insulin) (2000u)
[0047] Epidermal Growth Factor (5mg)
[0048] Silver Sulfadiazine (10g)
[0049] Triethanolamine (20g)
[0050] Stearic acid (100g)
[0051] Glycerin (50g)
[0052] Lanolin (20g)
[0053] White Vaseline (150g)
[0054] Ethylparaben (1g)
[0055] Add distilled water to 1000g
[0056] Preparation method: the oil phase is stearic acid, lanolin, white petrolatum and ethyl paraben; the water phase is triethanolamine, glycerin and water; the water phase and the oil phase are stirred and heated to 70-80 ℃ respectively, and then the oil phase The aqueous phase was added slowly while stirring well. When the temperature drops below 40°C, insulin, epidermal growth factor and silver sulfadiazine are added, and the mixture is fully stirr...
Embodiment 3
[0058] Example 3 consists of the active ingredients of the formulation by the following weights:
[0059] Insulin (Bovine Insulin) (500u)
[0060] Epidermal Growth Factor (10mg)
[0061] Gentamicin (10g)
[0062] Triethanolamine (20g)
[0063] Stearic acid (100g)
[0064] Glycerin (50g)
[0065] Lanolin (20g)
[0066] White Vaseline (150g)
[0067] Ethylparaben (1g)
[0068] Add distilled water to 1000g
[0069] Preparation method: the oil phase is stearic acid, lanolin, white petrolatum and ethyl paraben; the water phase is triethanolamine, glycerin and water; the water phase and the oil phase are stirred and heated to 70-80 ℃ respectively, and then the oil phase Slowly add the water phase while stirring well; when the temperature drops below 40°C, add insulin, epidermal growth factor or gentamicin, stir well and grind evenly, and that's it. Pack it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com