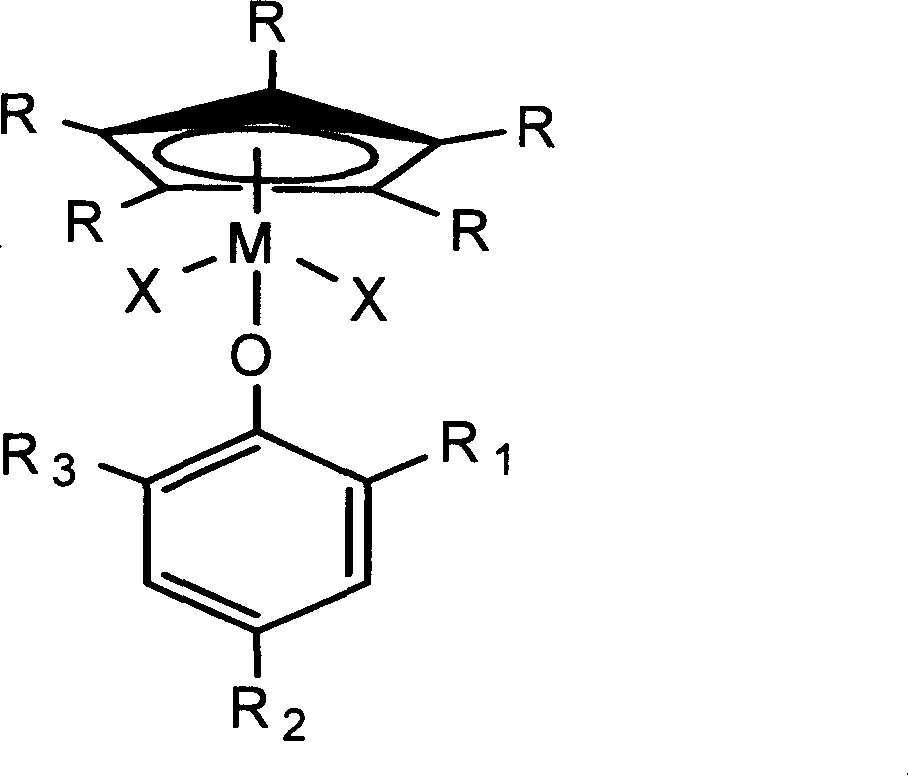
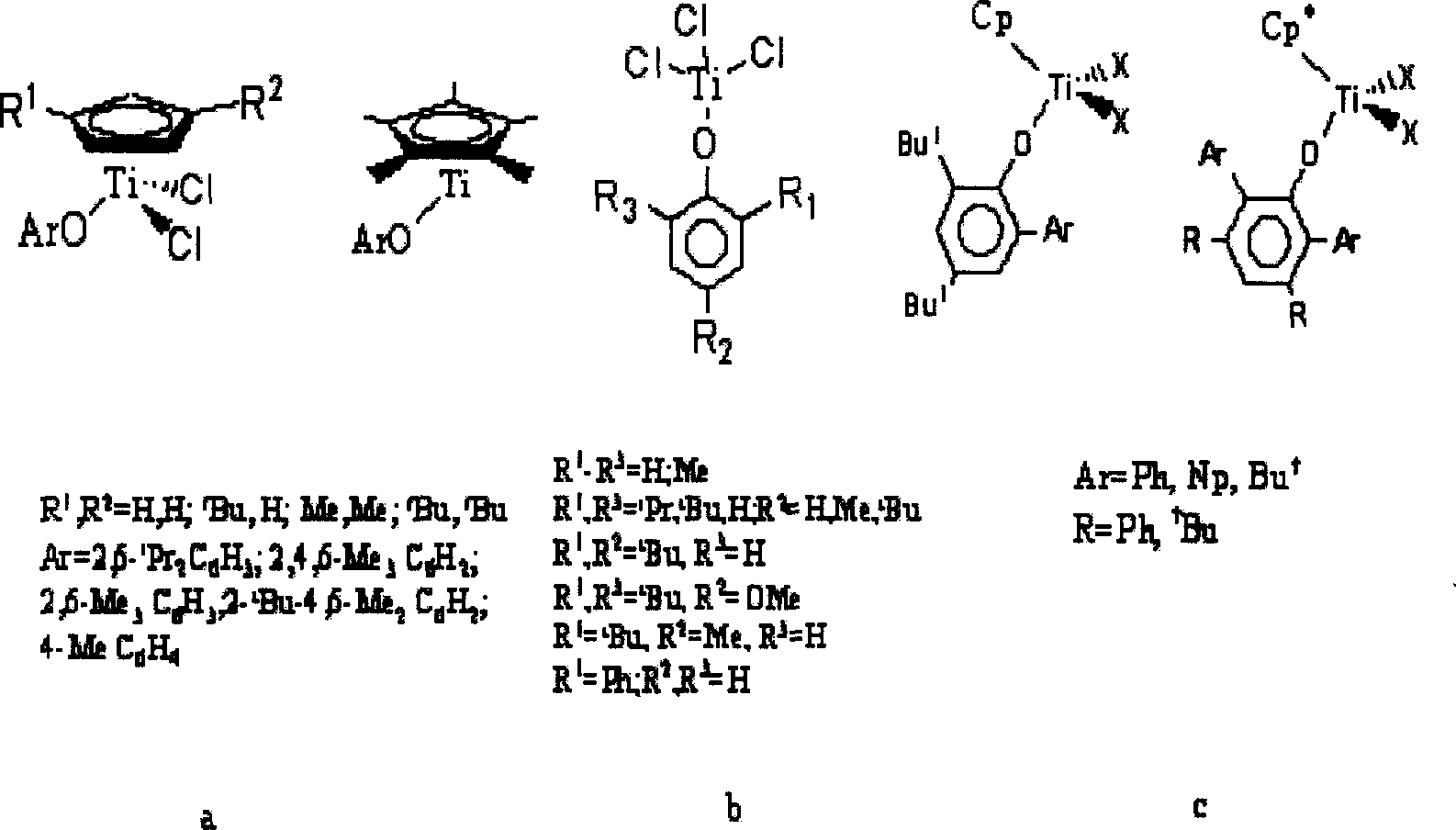
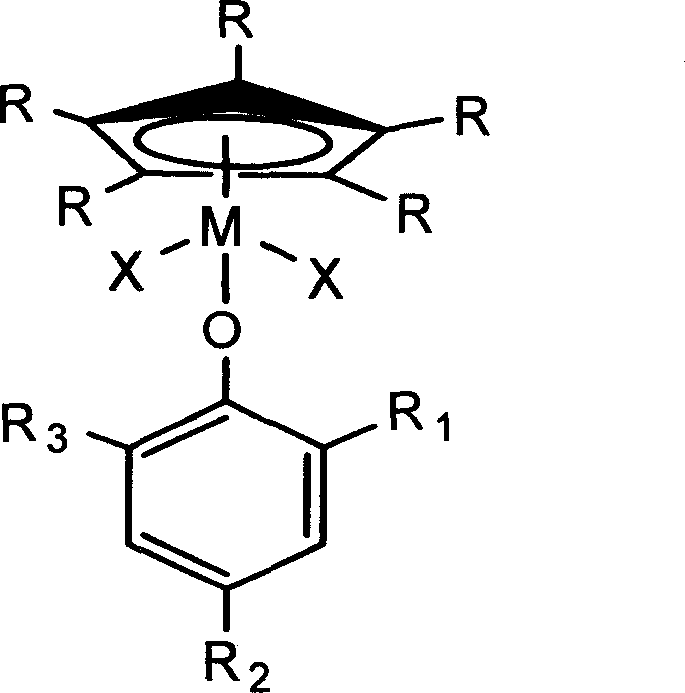
Monodentate compound containing phenoxy, preparation method and application thereof
A compound, phenoxy technology, applied in the field of phenoxy-containing monocene compounds and their preparation and application, can solve the problem of low catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1-Phenyl-2,3,4,5-tetramethylcyclopentadienyl-(2,4,6-tri-tert-butylphenoxy)-titanium dichloride catalyst
[0022] (1) Synthesis of 1-phenyl-2,3,4,5-tetramethylcyclopentenyl titanium trichloride
[0023]
[0024] In a 50ml dry ampoule, weigh 1.61g (8.13mmol) of 1-phenyl-2,3,4,5-tetramethyl-cyclopentadiene, add 20ml of anhydrous tetrahydrofuran, and slowly 7.5ml n-BuLi (9.00mmol) was slowly added dropwise. After one hour, the reaction system gradually rose to room temperature. After 8 hours of reaction, the solution turned into a white paste. 2.06ml of trimethylchlorosilane was slowly added to the reaction solution at -78°C, and then reacted at room temperature for 2 After 1 hour, the solution gradually turned bright yellow. The remaining trimethylchlorosilane and tetrahydrofuran solvents were distilled off under reduced pressure and extracted with hexane to obtain a yellow oil.
[0025] In a dry ampoule, under a nitrogen atmosphere, the above-mentioned trimethylsily...
Embodiment 2
[0033] The synthetic process of 1-phenyl-2,3,4,5-tetramethylcyclopentenyl titanium trichloride, reaction under ice-water bath condition, other operations are identical with embodiment 1, obtain catalyst product 0.78g, produce Rate 71.80%.
[0034] Catalyze ethylene polymerization according to the method in embodiment 1, obtain polymerization product 5.24g, catalyst activity is 2.21 * 10 6 gPE(molTi h) -1 , The weight-average molecular weight of polymer measured by GPC instrument is 270,000.
Embodiment 3
[0036] The synthesis process of 1-phenyl-2,3,4,5-tetramethylcyclopentenyl titanium trichloride, reacted at 40°C, and other operations were the same as in Example 1 to obtain 0.75g of product, the yield 69.15%.
[0037] Catalyze butene polymerization according to the method in embodiment 1, obtain polymerization product 3.84g, catalyst activity is 1.14 * 10 6 gPE(molTi h) -1 , The weight-average molecular weight of polymer measured by GPC instrument is 83000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com