Method for preparing antiform - 4 - acetoxy - 2 - methyl - butenoic aldehyde
A technology of acetoxy and crotonaldehyde, which is applied in the preparation of pentacyclic unsaturated carbon frame compounds and the field of preparation of trans-4-acetoxy-2-methyl-2-butenal, which can solve the problem of receiving The efficiency cannot reach the yield, the process cost is increased, and the production cost is increased, so as to achieve the effects of reducing reaction steps, easy recycling, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
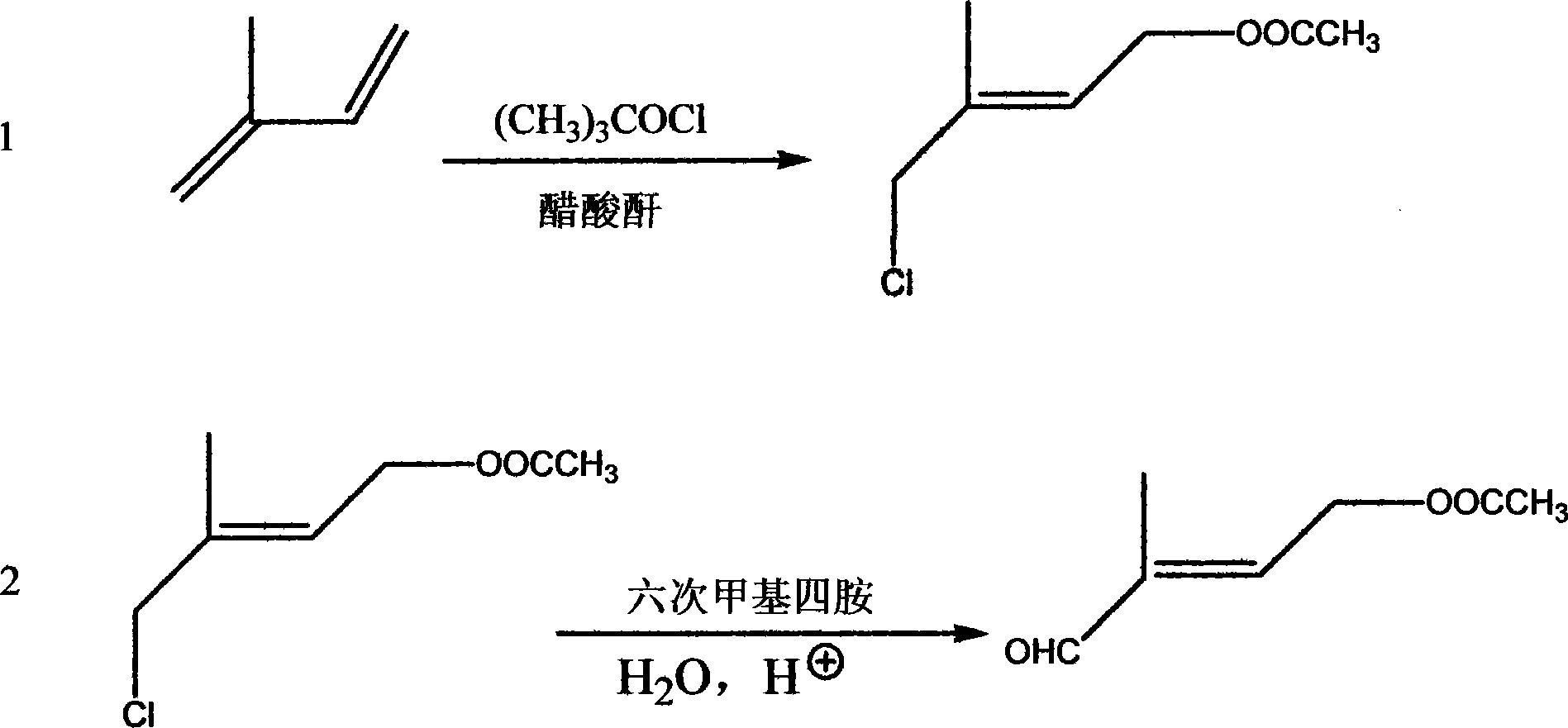
[0015] (1) Add 1 mol of isoprene to 2 mol of acetic anhydride solution, stir, cool to -10°C to 0°C, maintain this temperature, then add 1 mol of tert-butyl hypochlorite dropwise within 1.5 hours. After the dropwise addition, the temperature was raised to 80°C, and 0.05mol p-toluenesulfonic acid was added to react for 10 hours. After the reaction, 500ml ice water was added, and the organic phase was separated, dried with 20g anhydrous sodium sulfate, and filtered to remove the desiccant. At 5 mmHg, 0.8 mol of chloroester was distilled, with a content of 93%.
[0016] (2) Add 1mol of chloroester to 500ml of toluene, add 1mol of hexamethylenetetramine, keep the temperature at about 40°C, react for 8 hours, then add 500ml of water, heat up to 80°C, and then add 2mol of glacial acetic acid Add it dropwise within 2 hours, continue to react for 10 hours, divide the organic phase after the completion of the reaction, dry with 20g of anhydrous sodium sulfate, and under 0.5mmHg, distill...
example 2
[0018] (1) Add 1 mol of isoprene to a solution of 2 mol of acetic anhydride, stir, cool to -10-0°C, maintain this temperature, then add 1 mol of tert-butyl hypochlorite dropwise within 1.5 hours. After the dropwise addition, the temperature was raised to 80°C, and 0.1mol sulfuric acid was added to react for 10 hours. After the reaction, 500ml of ice water was added, the organic phase was separated, dried with 20g of anhydrous calcium chloride, and the desiccant was filtered off. At 5 mmHg, 0.81 mol of chloroester was distilled, with a content of 93%.
[0019] (2) Add 1 mol of chloroester to 500 ml of acetonitrile, add 1 mol of hexamethylenetetramine, keep the temperature at about 40°C, react for 8 hours, then filter, add 500 ml of water to the filter cake to dissolve, then add 500 ml of 1,2- Ethylene dichloride was heated up to 80°C, and then 2mol glacial acetic acid was added dropwise within 2 hours, and the reaction was continued for 6 hours. After the reaction was completed...
example 3
[0021] (1) Add 1 mol of isoprene to 2 mol of acetic anhydride solution, stir, cool to -10-0°C, maintain this temperature, then add 1 mol of tert-butyl hypochlorite dropwise within 1.5 hours. After the dropwise addition, raise the temperature to 80°C, add 0.1mol nitric acid to react for 10 hours, add 500ml ice water after the reaction, separate the organic phase, dry it with 20g anhydrous sodium sulfate, and filter off the desiccant. At 5 mmHg, 0.78 mol of chloroester was distilled, with a content of 91%.
[0022] (2) Add 1 mol of chloroester to 500 ml of petroleum ether, add 1 mol of hexamethylenetetramine, keep the temperature at about 40°C, react for 8 hours, then add 500ml of water, heat up to 90°C, and then add 2 mol of ice Acetic acid was added dropwise within 2 hours, and the reaction was continued for 6 hours. After the reaction, the organic phase was separated, dried with 20 g of anhydrous sodium sulfate, and distilled at 0.5 mmHg to obtain 0.74 mol of pentacarbonaldeh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com