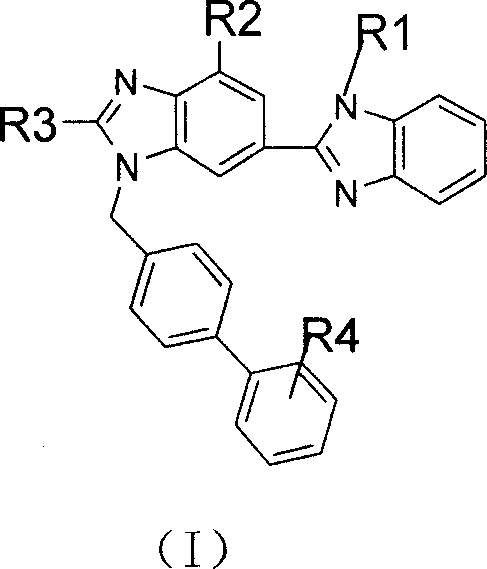
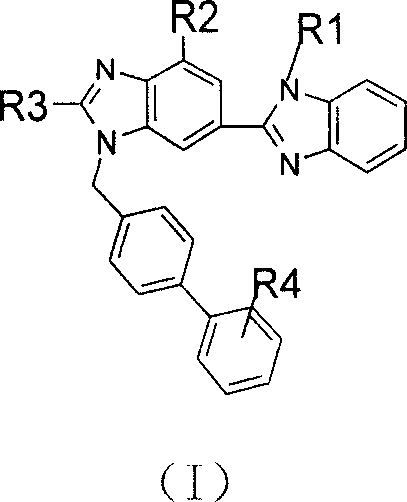
Bibenzimidazole derivative with PPARgamma exciting agent activity and application thereof
A technology of benzimidazole and biphenyl is applied to bisbenzimidazole derivatives with PPARγ agonist activity and their application fields, which can solve the problems of adipose tissue surge, bone marrow fatty acid change, and patient weight gain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
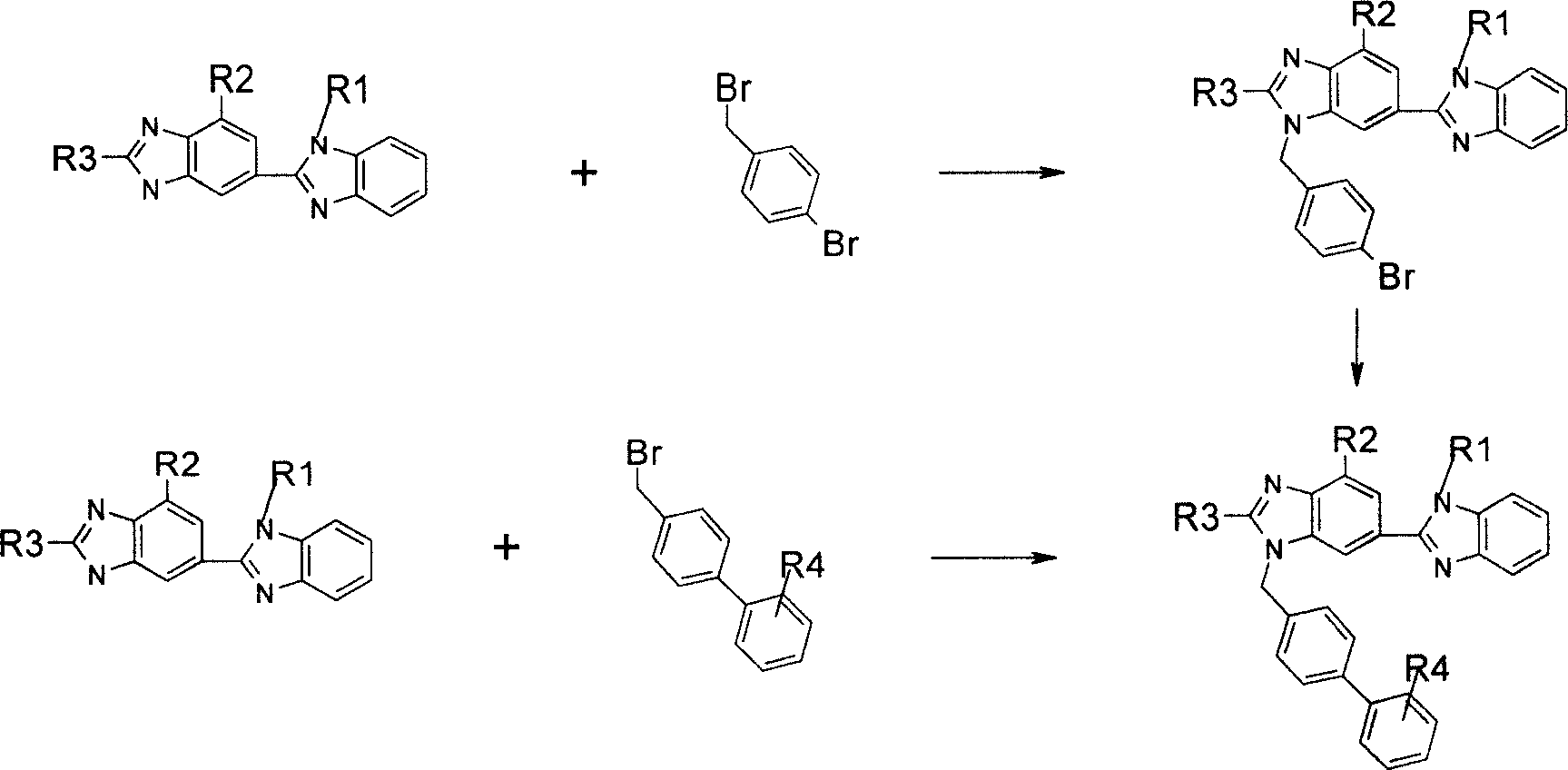
[0049] Example 1: 4'-[(2-n-propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-benzimidazol-1-yl)-methyl]- Biphenyl-2-methanol
[0050]
[0051] Dissolve 608 mg of bisimidazole, 2 mmol, in 30 ml of anhydrous DMF, add 88 mg of 60% NaH under stirring at room temperature, and then add 610 mg of biphenyl raw material. After reacting for 6 h, TLC monitored that the reaction was complete. Dilute the system with a large amount of ethyl acetate, wash with cold water, dry the organic phase, and purify the product. Yield 100%.
[0052] 1H NMR (400Mz, CDCl3): δ7.82-7.78 (2H, m), 7.54-7.45 (2H, m), 7.44-7.33 (3H, m), 7.32-7.21 (5H, m), 7.09 (2H, d, J=8.05Hz), 5.45(2H,s), 3.81(3H,s), 3.57(3H,s), 2.94(2H,t, J=7.78Hz), 2.77(3H,s), 1.95- 1.80(2H, m), 1.05(3H, t, J=7.41Hz).
[0053] Dissolve the above product in anhydrous THF and add 100mg LiAlH 4 Powder, the reaction was completely converted after 1h. Add a certain amount of ethyl acetate, wash with brine, dry the organic phase, concentr...
Embodiment 2
[0055] Example 2: 4'-[(2-n-propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-benzimidazol-1-yl)-methyl]- Biphenyl-4-methoxy
[0056]
[0057] Dissolve 3.04 g of bis-imidazole in 30 ml of DMF, add 410 mg of 60% NaH with stirring at room temperature, add 2.50 g of p-bromobenzyl bromide solid, and react overnight at 60°C. The system was diluted with water, extracted three times with ethyl acetate, the organic phases were combined, and washed once with water. Purify by dry chromatography (developing solvent: dichloromethane / methanol=10:1). A viscous product was obtained, which was recrystallized from ethyl acetate to obtain a white granular crystalline product.
[0058] Take 237mg, 0.5mmol of the above crystals, p-methoxyphenylboronic acid 88mg K 2 CO 3 Dissolve the solid powder together in 15ml of THF / EtOH (1:1) mixed solution, add catalyst Pd (PPh 3 ) 4 60mg, stirred and heated to 60°C for reaction. The color change of the system is first yellow, then indigo, and then in...
Embodiment 3
[0060] Example 3: 4'-[(2-n-propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-benzimidazol-1-yl)-methyl]- Methyl biphenyl-4-carboxylate
[0061]
[0062] Take 480mg, 1.01mmol of bisimidazole protected by bromobenzyl group, 200mg of methyl p-formate phenylboronic acid, and K2CO3 solid powder 276mg, dissolve in 30ml of THF / EtOH (1:1) mixed solution, slowly add catalyst Pd (PPh3 ) 4116mg, stirred and heated to 60°C for reaction. The color of the system gradually darkened, and the reaction was terminated after 1 h. The solvent was removed by concentration under reduced pressure, the residue was dissolved in ethyl acetate, washed with NaHCO3 and NaCl solution, dried and purified by chromatography to obtain a white solid product. (Developer: dichloromethane / methanol=20:1)
[0063] 1H NMR (400Mz, CDCl3): δ8.07 (2H, d, J=8.43Hz), 7.80-7.77 (1H, m), 7.61-7.52 (4H, m), 7.41-7.26 (5H, m), 7.14 (2H, d, J=7.88Hz), 5.46(2H, s), 3.92(3H, s), 3.80(3H, s), 2.93(2H, t, J=7.79Hz), 2.78(3H, s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com