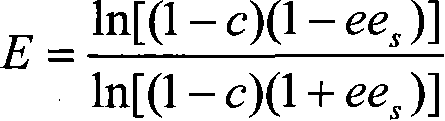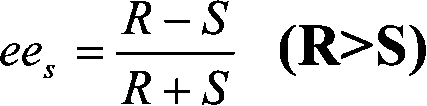Method for preparing S-ibuprofen and S-ibuprofen ester by biological catalysis
A technology of ibuprofen axetil and mixture, applied in the field of high optical purity S-ibuprofen and S-ibuprofen axetil, can solve the problems of unsatisfactory, low catalytic conversion rate stereoselectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
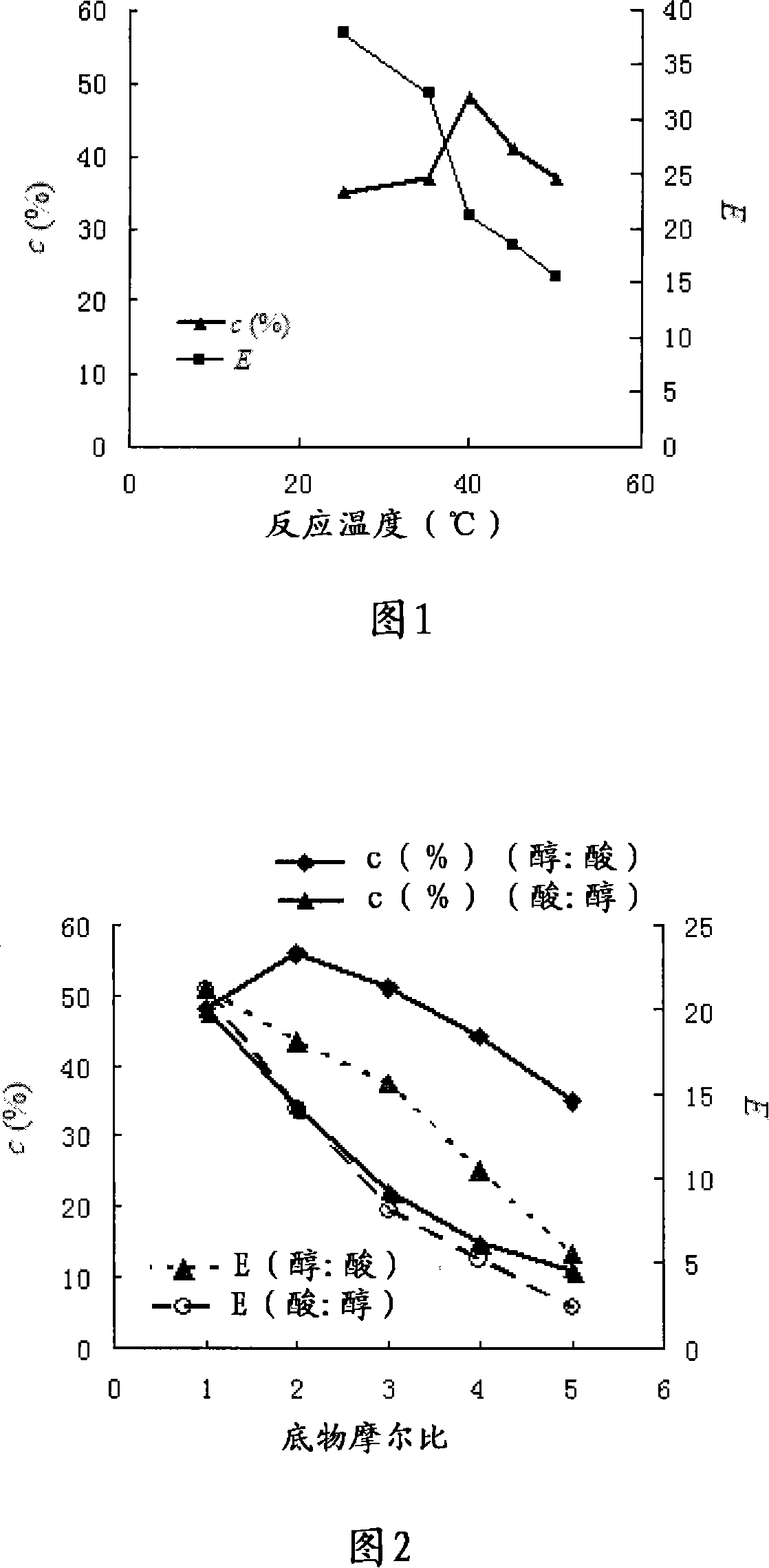
Image
Examples
preparation example Construction
[0017]The preparation of the immobilized enzyme of the present invention can be referred to the applicant's co-pending Chinese invention patent application No. 200510112638.5. In addition, the preparation of crude enzymes, immobilized enzymes, modified enzymes, and enzyme fermentation broths used in the present invention are also taught in the following documents: Tan TW, Zhang M, Wang BW, Ying CH, Deng L. Screening of high lipase producing Candida sp. and production of lipase by fermentation. Process Biochem 2003, 39(4): 459-65; Mingrui Yu, Shaowei Qin, Tianwei Tan, Purification and characterization of the extracellular lipase Lip2 from Yarrowia lipolytica, Process Biochemistry 4 (2007) 384-391; Kaili Nie, Feng Xie, Fang Wang, TianweiTan, Lipase catalyzed methanolysis to produce biodiesel: Optimization of the biodiesel production. Journal of Molecular Catalysis B: Enzymatic 43(2006) 142-147; and Chinese Invention Patent Application No. 02017614. , its publication number is CN...
Embodiment 1
[0041]In a 100ml Erlenmeyer flask with a stopper, 10ml of n-hexane solution of racemic ibuprofen (purchased from Sigma, the same below) with a concentration of 0.03mol / L, 0.3mmol of ethanol, and 0.2g of immobilized enzyme were sequentially added. The immobilized enzyme refers to the applicant's co-pending Chinese invention patent application No.200510112638.5 and Tan TW, Zhang M, Wang BW, Ying CH, Deng L.Screening of high lipase producing Candida sp.and production of lipase byfermentation.Process Prepared by the method in Biochem 2003; 39(4): 459-65, hereinafter the same. The reaction mixture was placed on a shaker at 40°C, shaken and incubated at a speed of 180 rpm for 24 hours to obtain a mixture containing racemic ibuprofen, S-ibuprofen ethyl ester, R-ibuprofen ethyl ester mixture. Take 20 microliters of the above mixture for analysis, the analysis method is as follows:
[0042] Detection by high performance liquid chromatography (chiral column: CHIRALCEL OB ~ H, 15cm × 4...
Embodiment 2
[0050] In a 100 ml Erlenmeyer flask with a stopper, 10 ml of a 0.03 mol / L racemic ibuprofen n-hexane solution, 0.6 mmol of ethanol, and 0.2 g of immobilized enzyme were sequentially added. Reaction, detection, separation and hydrolysis under the same conditions as in Example 1, the results are as follows: the conversion rate c is 0.396, the excess value ees=0.606 of the substrate enantiomer, and the stereoselectivity E=47.22 of the enzyme (the maximum of the enzyme) Preferable selectivity is S type).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com