Tsiklomitsin molecular engram polyalcohol and uses of the same
A molecular imprinting and tetracycline technology, applied in the direction of material separation, analysis materials, instruments, etc., can solve the problem of high price and achieve the effect of high purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 2.0 g of template tetracycline hydrochloride molecule (China National Institute for Pharmaceutical and Biological Control), 4.0 g of functional monomer methacrylic acid (MAA) (analytical purity, Tianjin Komiou Chemical Reagent Center), and cross-linking agent ethylene glycol dimethacrylate (EDMA) 60.0g (Fluka Company), plus 0.20g initiator azobisisobutyronitrile, dissolve and mix the above reaction mixture in a 50KHz ultrasonic cleaning machine, and add it to the reactor for in-situ polymerization. The temperature is controlled between 75 and 80°C, and the reaction time is 20 hours, to obtain a hard, bulky yellow polymer.
[0020] After the polymerization reaction, the synthesized tetracycline molecularly imprinted polymer was taken out of the reactor, ground and passed through a 100-mesh sieve. The obtained powder was extracted with 80% methanol reflux to remove the template tetracycline hydrochloride molecule (80% determined by liquid chromatography). It takes about ...
Embodiment 2
[0023] Take the template tetracycline hydrochloride molecule 4.0g (analytical grade, China National Institute for Pharmaceutical and Biological Control), functional monomer methacrylic acid (MAA) 6.0g (analytical grade, Tianjin Komiou Chemical Reagent Center), cross-linking agent dimethacrylic acid B EDMA 80.0g (Fluka Company), plus 0.4g initiator azobisisobutyronitrile, dissolve and mix the above reaction mixture in a 50KHz ultrasonic cleaning machine, and add it to the reactor at 366nm UV The in-situ polymerization was carried out under the light lamp irradiation, and the reaction time was 24 hours to obtain a hard and bulky yellow polymer.
[0024] After the polymerization reaction, the synthesized tetracycline molecularly imprinted polymer was taken out of the reactor, ground and passed through a 100-mesh sieve. The obtained powder was extracted with 80% methanol reflux to remove the template tetracycline hydrochloride molecule (80% determined by liquid chromatography). It tak...
Embodiment 3
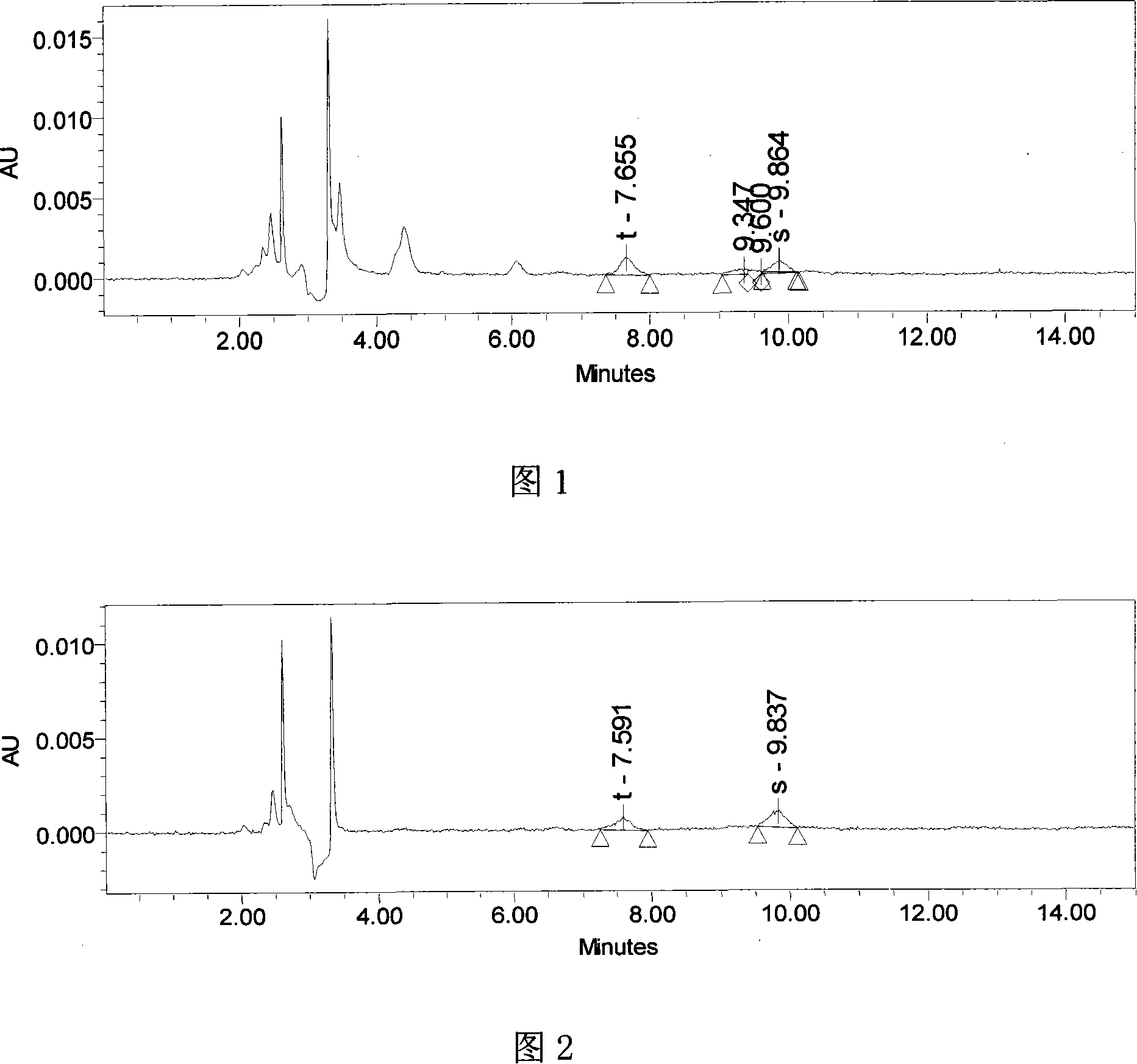
[0027] The tetracycline molecularly imprinted solid phase extraction column obtained in Examples 1 and 2 was used to perform sample pretreatment on tetracycline and oxytetracycline spiked pork as follows:
[0028] 1. Take 5.0g of minced tetracycline and oxytetracycline spiked pork (0.1mg / kg each of tetracycline and oxytetracycline), homogenize it with 20mL0.01mol / L EDTA-2Na, add 2mL1∶1(V:V ) Aqueous trichloroacetic acid solution, centrifuged at 20000 rpm for 10 minutes, and then take the supernatant, which is the sample to be tested.
[0029] 2. Take a tetracycline molecularly imprinted solid phase extraction column (200mg / 6mL), first use 5mL methanol, 5mL water, 5mL0.01mol / LEDTA-2Na solution to pass through the column activation column, then pass all the above test samples through the column, and then use 5mL Wash with water, vacuum dry the water in the column, and finally collect with 5mL methanol. The methanol phase is dried under nitrogen at 50℃, and dissolved by adding 1mL0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com