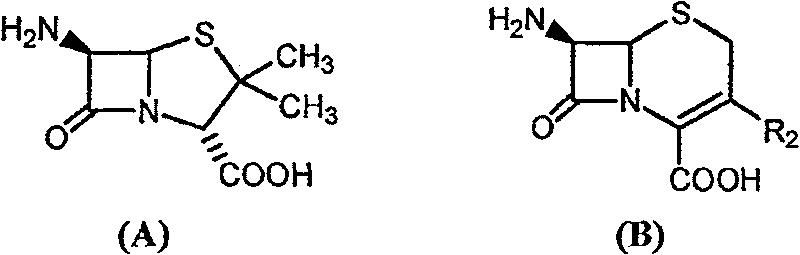Method for enzymatically synthesizing beta-lactam antibiotic in mixed system of water and organic medium
A lactam and medium technology, applied in the field of enzyme-catalyzed synthesis of β-lactam antibiotics, can solve the problems of increased yield, difficult control of acyl donor side chains and product hydrolysis, and improved yield, reduced deprivation ability, and side effects. The effect of response inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Enzyme-catalyzed synthesis of amoxicillin in tert-amyl alcohol containing 2% water
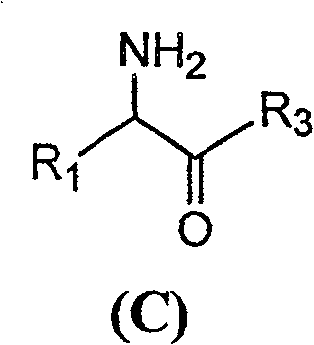
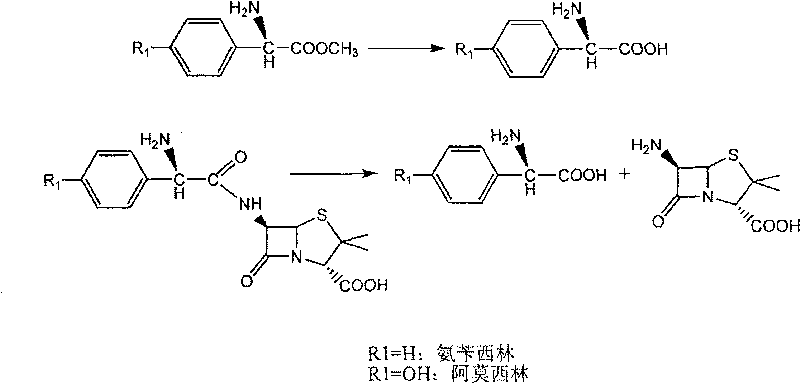
[0046] With 2.16 grams of 6-APA (10mmol) and 3.6 grams of D-hydroxyphenylglycine methyl ester (20mmol) (general formula (C) compound, wherein R1 is p-hydroxyphenyl, R3 is methoxy, abbreviated D-HPGM ) into 98ml of analytically pure tert-amyl alcohol through molecular sieve removal of water. Then, add 2ml of water to the reaction system, place the reaction mixture in a constant temperature incubator and vibrate for 5 minutes to make each substance well dispersed, add 0.1g / ml of penicillin G acylase, and react at 10°C for 12 hours , 88% of the amoxicillin productive rate determined by liquid chromatography, the molar ratio selectivity of the synthetic product of amoxicillin and the hydrolyzate of D-hydroxyphenylglycine methyl ester is 1.6 (compared with, for the aqueous medium under common conditions is around 0.6).
Embodiment 2
[0047] Example 2 Enzyme-catalyzed synthesis of amoxicillin in tert-amyl alcohol containing 10% water
[0048] 2.16 g of 6-APA (10 mmol) and 3.6 g of D-HPGM (20 mmol) were added to 90 ml of analytically pure tert-amyl alcohol which was dewatered by molecular sieves. Then, add 10ml of water to the reaction system, place the reaction mixture in a constant temperature incubator and vibrate for 5 minutes to make each substance well dispersed, add 0.1g / ml of penicillin G acylase, and react at 10°C for 8 hours , liquid chromatographic determination of amoxicillin productive rate 83%, the molar ratio selectivity of the synthetic product of amoxicillin and the hydrolyzate of D-hydroxyphenylglycine methyl ester is 1.4 (compared with, for the aqueous medium under common conditions is around 0.6).
Embodiment 3
[0049] Example 3 Enzyme-catalyzed synthesis of ampicillin in ethyl acetate containing 2% water
[0050] 2.16 grams of 6-APA (10mmol) and 3.3 grams of D-phenylglycine methyl ester (20mmol) (general formula (C) compound, wherein R1 is phenyl, R3 is methoxyl, abbreviated D-PGM) is added to 98ml Pure ethyl acetate (<0.1% water content) was analyzed. Then, add 2ml of pH6.5 phosphate buffer solution to the reaction system, place the reaction mixture in a constant temperature incubator and shake for 5 minutes, add 0.1g / ml of penicillin G acylase, and react at 10°C for 8 hours , liquid chromatographic determination of ampicillin yield 93%, ampicillin synthesis product and the molar ratio selectivity of the hydrolysis product of D-phenylglycine is 2.0 (compared with about 1.2 for the aqueous medium under common conditions).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com