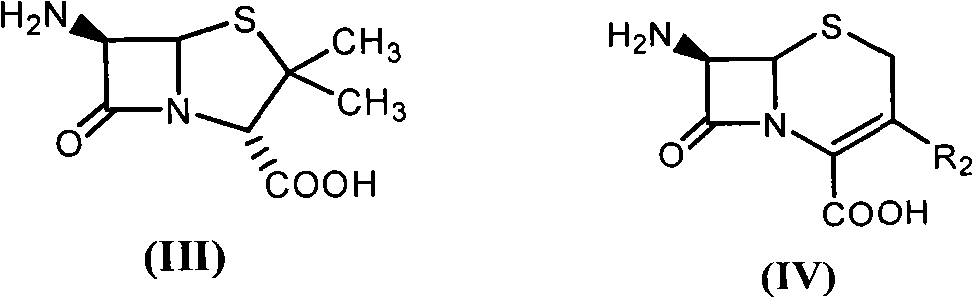Method for enzymatically synthesizing beta-lactam antibiotic in organic solvent
An organic solvent and lactam technology, which is applied in the field of preparation of beta-lactam antibiotics, can solve the problems of difficulty in controlling the hydrolysis of acyl donor side chains and products, and increase the yield, and achieves the improvement of the yield, the reduction of by-products, the realization of The effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 Enzyme-catalyzed synthesis of ampicillin in anhydrous ethyl acetate
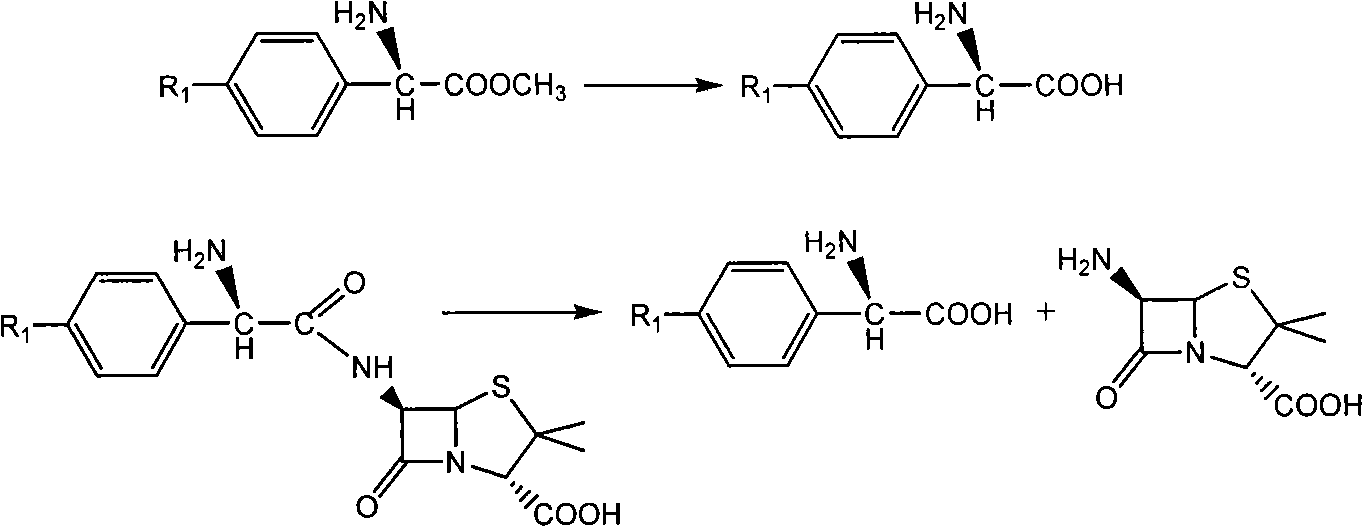
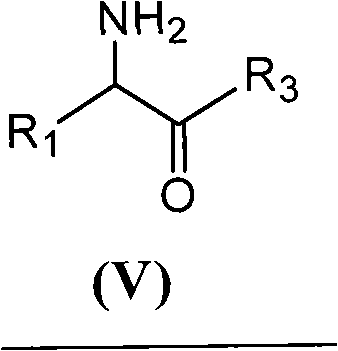
[0049] With 2.16 grams of 6-aminopenicillanic acid (10mmol) (general formula (III) compound, abbreviated 6-APA) and 3.3 grams of D-phenylglycine methyl ester (20mmol) (general formula (V) compound, wherein R is Phenyl, R3 is methoxy, abbreviated as D-PGM) was added to ethyl acetate which was dehydrated by molecular sieve to form a mixture with a total volume of 100ml. Then, the mixture of the reaction was placed in a constant temperature incubator to vibrate for 5 minutes, and the penicillin G acylase of 10.0 grams (1180 IU) was added, and reacted for 12 hours at 15° C., and the conversion rate of the antibiotic nucleus reached 90% as determined by liquid chromatography. The ampicillin yield was 88%, and the molar ratio selectivity of the synthetic product of ampicillin and the hydrolyzed product of D-phenylglycine was 2.5 (compared with about 1.2 for the reaction in aqueous medium under ...
Embodiment 2
[0050] Embodiment 2 Enzyme-catalyzed synthesis of amoxicillin in anhydrous tert-amyl alcohol
[0051] With 2.16 grams of 6-APA (10mmol) and 3.6 grams of D-hydroxyphenylglycine methyl ester (20mmol) (general formula (V) compound, wherein R1 is p-hydroxyphenyl, R3 is methoxy, abbreviated D-HPGM ) was added to tert-amyl alcohol which was dehydrated by molecular sieve to form a mixture with a total volume of 100ml. Then, place the reaction mixture in a constant temperature incubator and vibrate for 5 minutes so that each substance is well dispersed, add 20.0 grams (2360 IU) of penicillin G acylase, react at 15°C for 20 hours, and determine the antibiotic mother by liquid chromatography. The nuclear conversion rate reaches 86%, the amoxicillin productive rate is 85%, and the molar ratio selectivity of the synthetic product of amoxicillin and the hydrolysis product of D-p-hydroxyphenylglycine methyl ester is 1.8 (by comparison, for water under normal conditions It is about 0.6 when...
Embodiment 3
[0052] Embodiment 3 Enzyme-catalyzed synthesis of amoxicillin in isopropanol-n-octane mixed solvent
[0053] 2.16 g of 6-APA (10 mmol) and 5.4 g of D-HPGM (30 mmol) were added to isopropanol-n-octane (50 / 50) through molecular sieves to form a mixture with a total volume of 100 ml. The reaction mixture was placed in a constant temperature incubator and shaken for 5 minutes so that each substance was well dispersed. Add the penicillin G acylase of 20.0 grams (2360IU), react at 15 ℃ for 20 hours, the liquid phase chromatographic determination antibiotic mother nucleus conversion rate reaches 94%, amoxicillin productive rate 92%, the synthetic product of amoxicillin and D - The molar ratio selectivity of the hydrolyzed product of p-hydroxyphenylglycine methyl ester is 1.5 (compared to about 0.6 for the reaction in aqueous medium under ordinary conditions).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com