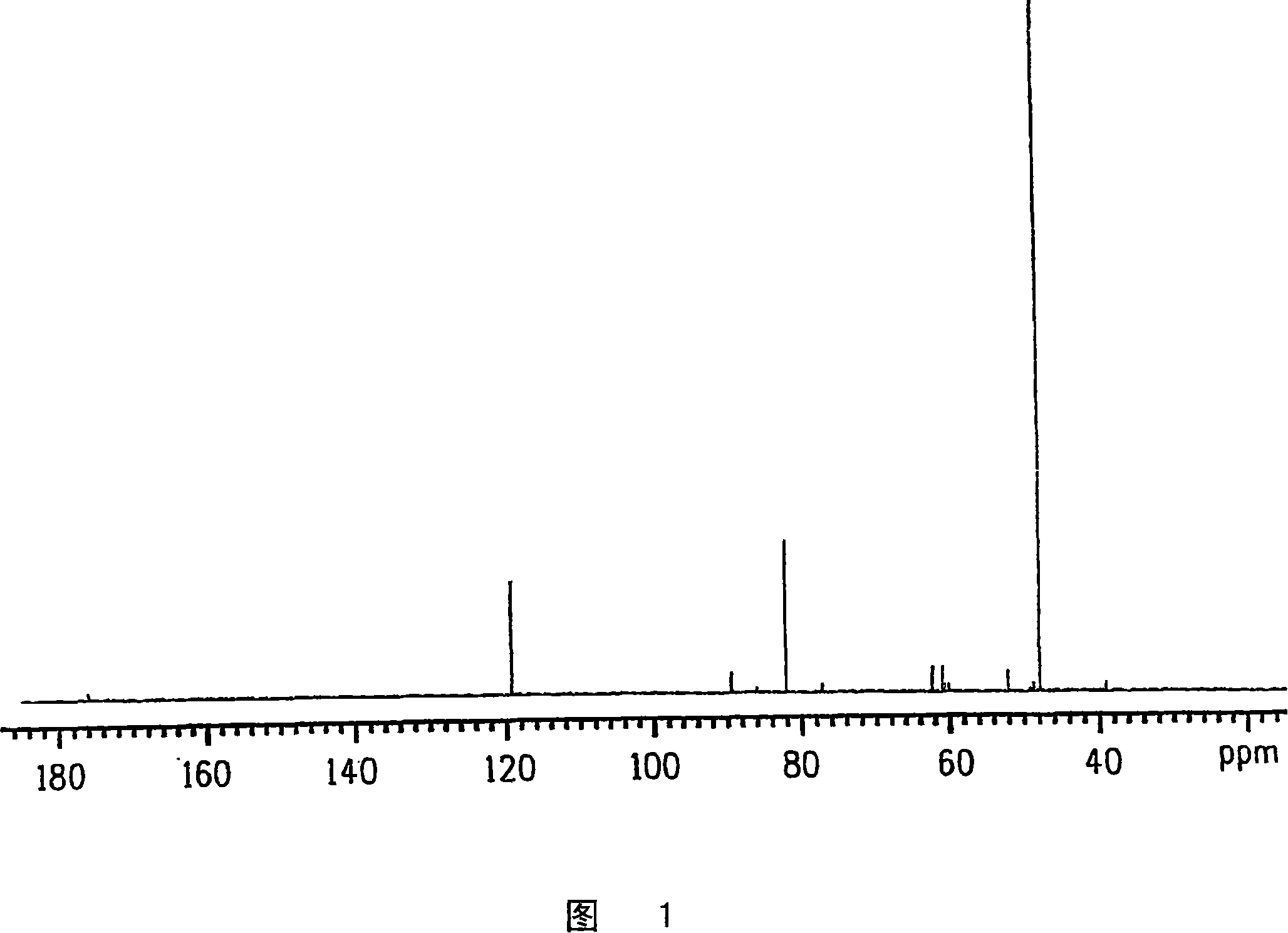
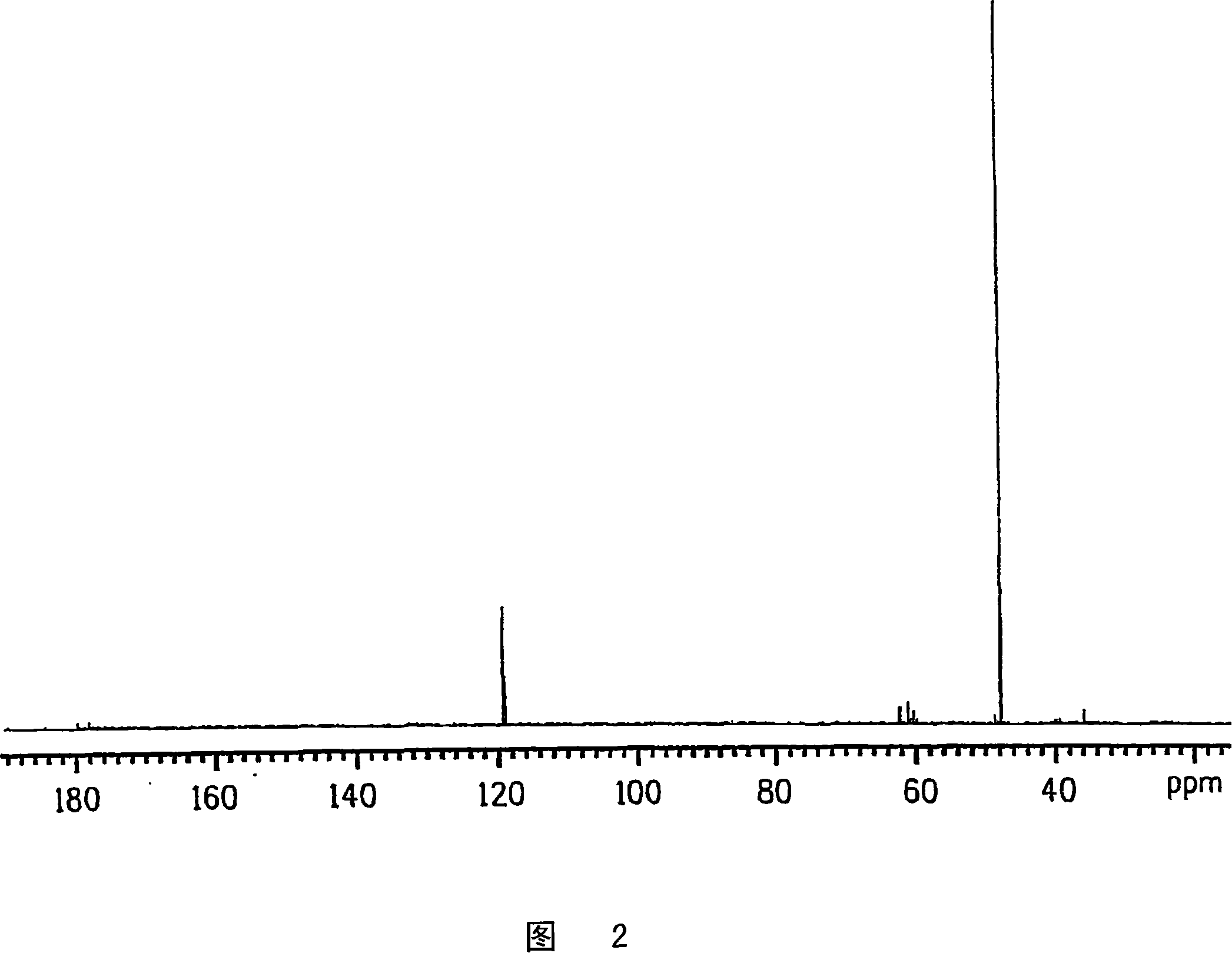
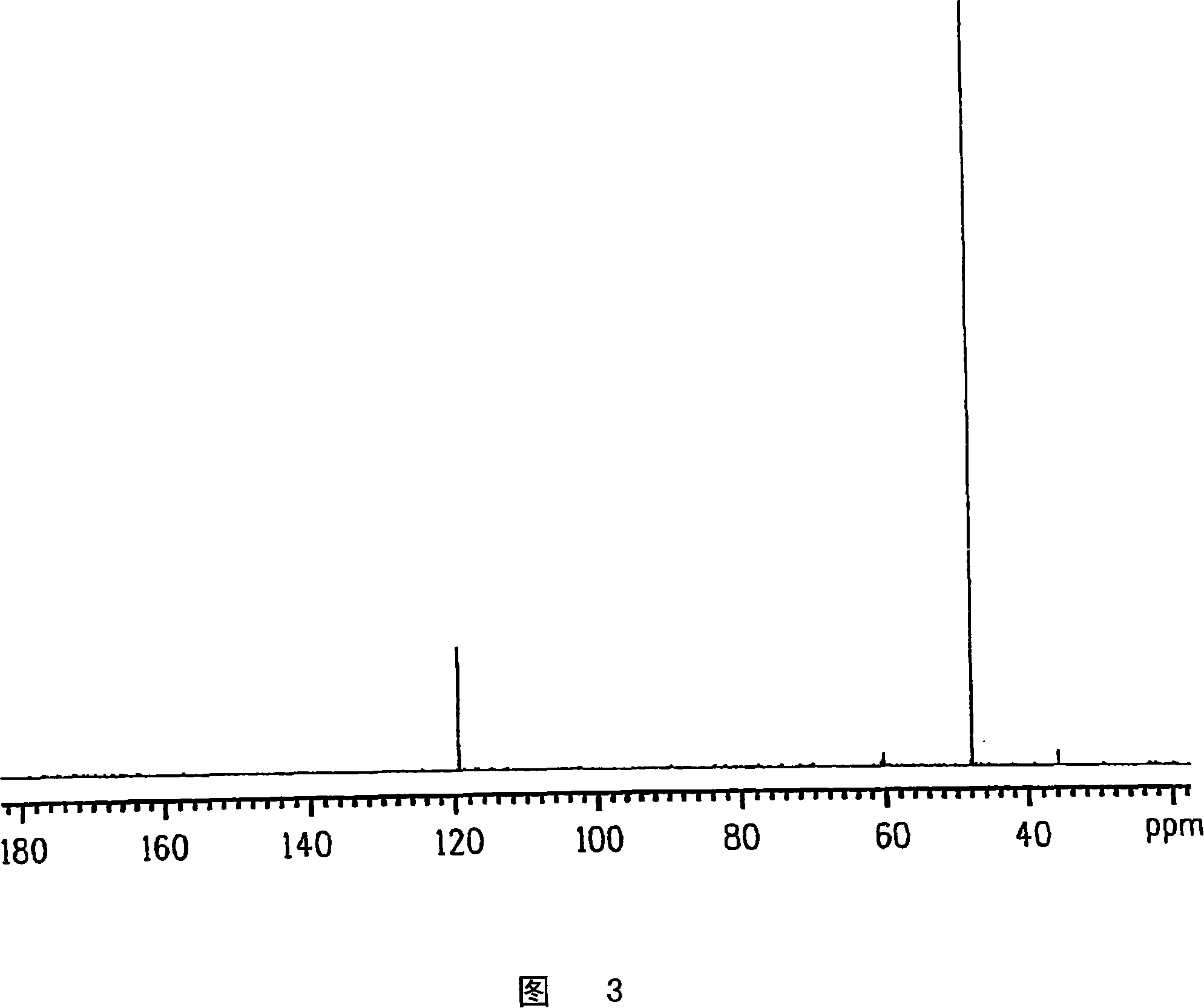
Process for producing glycolic acid from formaldehyde and hydrogen cyanide
A technology of glycolic acid and hydrogen cyanide, applied in chemical instruments and methods, cyanide reaction preparation, organic compound preparation, etc., can solve the problem of no nitrilase activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0342] Preheat 90% formaldehyde continuous feed
[0343] About 10.18 g of 52 wt% aqueous formaldehyde (<1% methanol, DuPont) was mixed with 12.81 g of water and the raw meal was heated to about 76°C for about 40 min until the mixture became a clear homogeneous liquid solution. The solution was cooled to room temperature, still a homogeneous liquid. Then, 0.16 mL of 16.7 wt% NaOH aqueous solution was added to the formaldehyde solution. 1.56 g of the as-synthesized solution (23 wt% formaldehyde) was placed in the autoclave and the remainder was used for continuous formaldehyde feed.
[0344] The reaction vessel equipped with stirring was placed in an oil bath maintained at 55°C. An approximately 12-inch section formaldehyde feed line (1 / 16" OD (approximately 1.6 mm) x 0.040" ID (approximately 1.02 mm)) placed directly before the reaction flask inlet was heated to 120°C, then heated for approximately 2.0 hours. The following reactants are each continuously pumped into the re...
Embodiment 2
[0350] Preheating 100% formaldehyde continuous feed
[0351] About 10.18 g of 52 wt% aqueous formaldehyde (<1% methanol, E.I. DuPont de Nemours) was mixed with 12.81 g of water and the raw meal was heated to about 76°C for about 40 min until the mixture became a clear homogeneous liquid solution. The solution was cooled to room temperature, still a homogeneous liquid. Then, 0.14 mL of 16.7 wt% NaOH aqueous solution was added to the formaldehyde solution. The resulting solution (23 wt% formaldehyde) was used for continuous formaldehyde feed.
[0352] A reaction vessel equipped with stirring was charged with a mixture of 0.18 g HCN in 3.4 g water and then placed in an oil bath maintained at 55°C. An approximately 12-inch section formaldehyde feed line (1 / 16" OD x 0.040" ID) placed directly before the reaction flask inlet was heated to 120°C, and the following reactants were each continuously pumped over a period of approximately 2.0 hours Into the reactor:
[0353] 4.41 mL...
Embodiment 3
[0358] Preheating 100% formaldehyde continuous feed
[0359] Approximately 14.20 g of 37 wt% formaldehyde in water (10-15% methanol, Acros Organics, Morris Plains, NJ) was mixed with 8.78 g of water and 0.14 mL of 16.7 wt% NaOH solution. The resulting solution (23 wt% formaldehyde) was used for continuous formaldehyde feed.
[0360] A reaction vessel equipped with stirring was charged with a mixture of 0.18 g HCN in 3.4 g water and then placed in an oil bath maintained at 55°C. An approximately 12-inch section formaldehyde feed line (1 / 16" OD x 0.040" ID) placed directly before the reaction flask inlet was heated to 120°C, and the following reactants were each continuously pumped over a period of approximately 2.0 hours Into the reactor:
[0361] 4.21 mL / hr of 50 wt% HCN in water (d=0.86 g / mL)
[0362] 7.67 mL / hr of 23 wt% aqueous formaldehyde, as described above (d = 1.07 g / mL).
[0363] After about 2.0 hours, the feeds were stopped, the autoclave was removed from the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com