Nonaqueous electrolyte for electrochemical energy storage device and electrochemical energy storage device making use of the same
A non-aqueous electrolyte and energy storage technology, applied in the direction of non-aqueous electrolyte batteries, electrolytic capacitors, electrochemical generators, etc., can solve the problems of damage to the protective film, difficulty in inserting lithium ions, and increased capacity degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
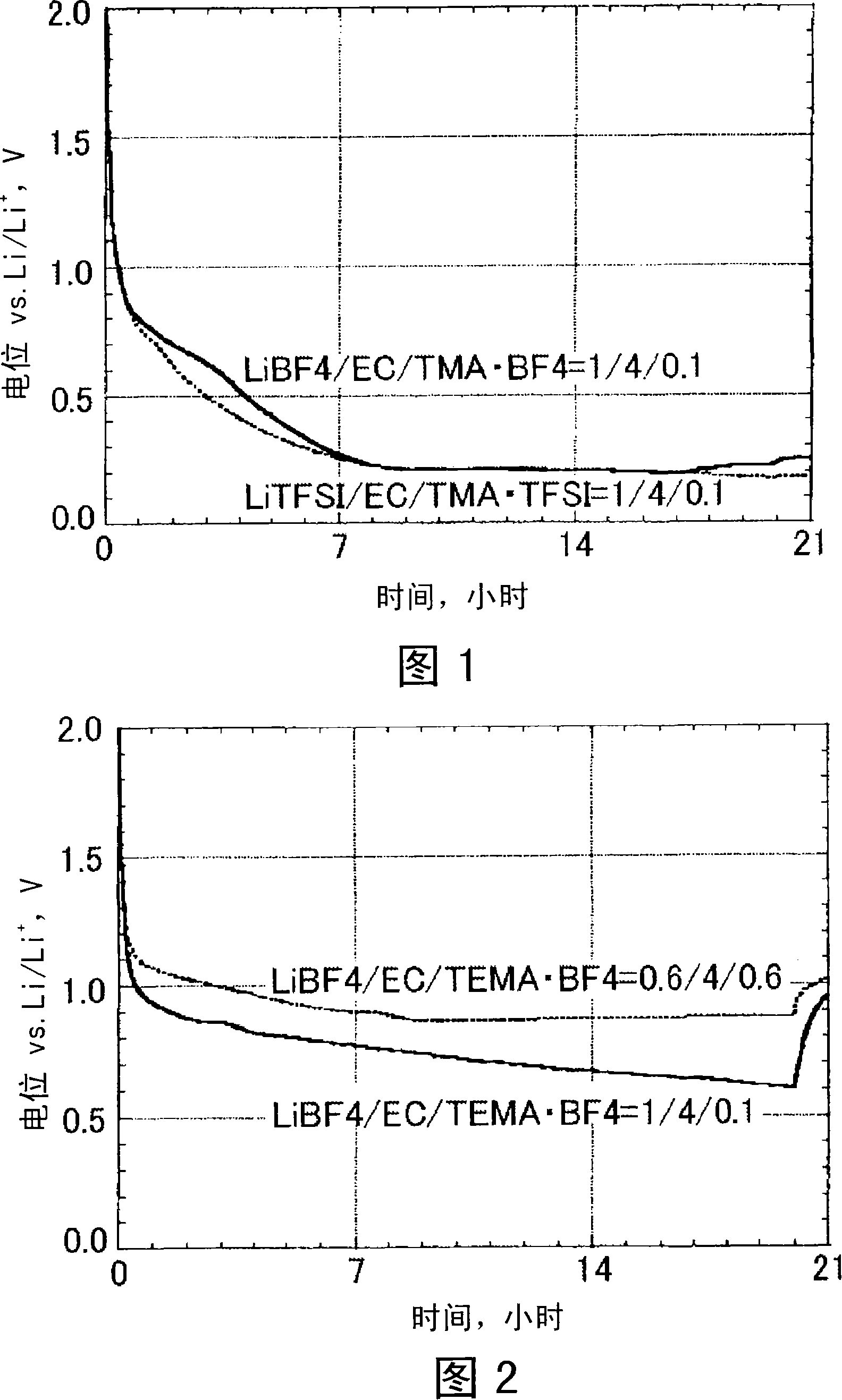
[0035] Artificial graphite powder is used as an anode material for intercalation / deintercalation of lithium ions during charge and discharge. The negative electrode plate was produced by the following method. First, 75 parts by mass of artificial graphite powder, 20 parts by mass of acetylene black as a conductive agent, 5 parts by mass of polyvinylidene fluoride resin as a binder, and dehydrated N-methyl-2-pyrrolidone as a dispersion solvent to mix. Next, the mixture was coated on one surface of a 20 μm thick copper foil current collector and dried to form an 80 μm thick active material layer. Then, the copper foil current collector formed with the active material layer was cut into a size of 35 mm × 35 mm, and a copper current collector plate with a lead wire thickness of 0.5 mm was welded to the obtained copper foil current collector by ultrasonic welding. On the body, thereby making the negative electrode plate.
[0036] In addition, the mixed LiBF 4 , EC and TMA·BF 4...
Embodiment 2
[0047] Use ethyl (TMEA ions), propyl (TMPA ions), butyl (TMBA ions), pentyl (TMPeA ions), hexyl (TMHA ions) with sequentially increasing alkyl chain lengths to replace one of the TMA ions Based on quaternary ammonium salts, the effect of alkyl chain length was studied. Anions are fixed as TFSI ions.
[0048] Each electrolyte solution was prepared by mixing LiTFSI, EC, and each quaternary ammonium salt at a molar ratio of 0.6 / 4 / 0.6.
[0049] In the same manner as in Example 1, a negative plate with artificial graphite powder was used as a test electrode, and the electrochemical intercalation of lithium ions into the artificial graphite powder was tested in each of the prepared electrolytes. Insertion conditions are set at 20°C, 0.03mA / cm 2 and 60mAh / g. After lithium ions are inserted into the artificial graphite powder, at 0.03mA / cm 2 The current flowed through the anode current, and the deintercalation of lithium ions from the artificial graphite powder was tested. The fi...
Embodiment 3
[0060] The evaluation was carried out for quaternary ammonium salts in which the quaternary ammonium cation was fixed as TMEA ion and as anion, respectively, with PF 6 -, BF 4 - , ClO 4 - , TFSI ion, BETI ion, MBSI ion, CHSI ion, BOB ion, CF 3 BF 3 - 、C 2 f 5 BF 3 - 、C 3 f 7 BF 3 - , (C 2 f 5 ) 3 PF 3 - . In addition, LiTFSI was used as a lithium salt.
[0061] Each electrolyte solution was prepared by mixing lithium salt, EC, and each quaternary ammonium salt at a molar ratio of 1 / 4 / 0.1.
[0062] In the same manner as in Example 1, a negative electrode plate having artificial graphite powder was used as a test electrode, and electrochemical intercalation of lithium ions into the artificial graphite powder was tested in each electrolyte solution. Insertion conditions are set at 20°C, 0.03mA / cm 2 and 60mAh / g. After lithium ions are inserted into the artificial graphite powder, at 0.03mA / cm 2 The current flowed through the anode current, and the deinterc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com