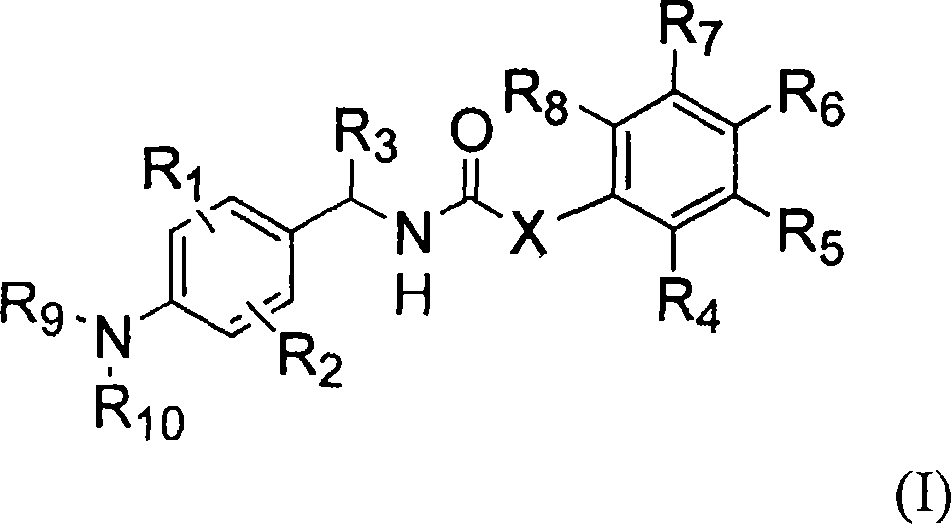
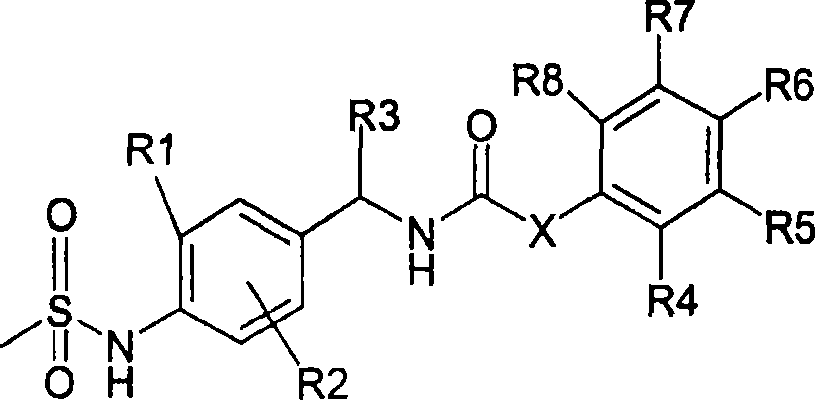
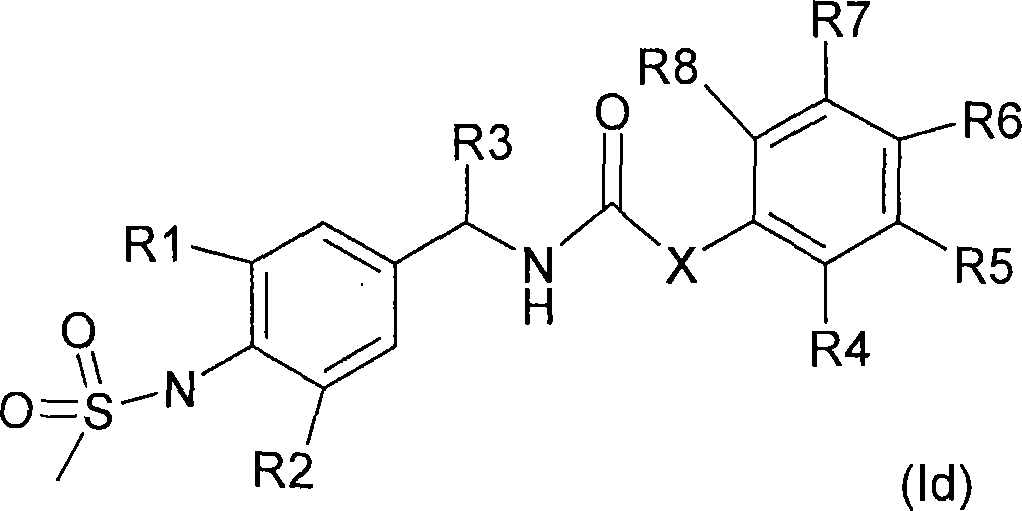
Novel compounds, isomer thereof, or pharmaceutically acceptable salts thereof as vanilloid receptor antagonist; and pharmaceutical compositions containing the same
A technology of compounds and isomers, applied in the field of vanilloid receptors, can solve problems such as nerve cell death and nerve function damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0362] Example 1: N-{4-[3-(4-tert-butylbenzyl)ureidomethyl]-2-vinylphenyl}methanesulfonamide
[0363]
[0364] Step 1: tert-Butyl (4-nitrobenzyl)carbamate
[0365] 4-Nitrobenzylamine HCl (1 g, 5.302 mmol, 1 eq.) was placed in a 100 ml round bottom flask and dissolved in saturated solution (NaHCO 3 :CH 2 Cl 2 = 1:1). To this solution was added di-tert-butyl dicarbonate (3.66ml, 15.906mmol, 3eq.) and stirred for 3 hours. After the completion of the reaction was confirmed by TLC, the reaction solution was extracted with dichloromethane, washed with water (2 times) and brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. The obtained liquid was subjected to column chromatography (n-hexane / ethyl acetate=10 / 1) to yield a light green solid (1.3171 g).
[0366] IR (KBr flake precipitate, cm -1 ): 3330, 3080, 301, 2984, 2916, 1687, 1513;
[0367] 1 H NMR (400MHz, CDCl 3 ): 8.11(d, 2H, J=8.4Hz), 7.37(d, 2H, J=8.4Hz), 4.99(bs, 1H), 4.37(d, 2H, J...
Embodiment 2
[0389] Example 2: N-{4-[3-(4-tert-butylbenzyl)ureidomethyl]-2-fluoro-6-vinylphenyl}methanesulfonamide
[0390]
[0391] Step 1: 4-Aminomethyl-2-fluoro-6-iodoaniline
[0392]A 50 ml double necked round bottom flask was filled with argon and a solution of 4-amino-3-fluoro-5-iodo-benzonitrile (84.4 mg, 0.322 mmol, 1 eq.) in tetrahydrofuran was placed in the flask, followed by cooling to 0 ℃. To this solution was added borane-THF complex solution (1.0 M in THF, 0.64 ml, 0.644 mmol, 2 eq.). The temperature of the reaction mixture was raised to room temperature. The reaction mixture was heated and refluxed. After the completion of the reaction was confirmed by TLC, 5% HCl was added to the solution and stirred for 20 minutes. The resulting solution was basified using 1N KOH, extracted with ether, washed with brine, passed through Na 2 SO 4 dry. The resulting liquid was concentrated under reduced pressure to yield a light yellow solid (78.4 mg, 80.5%).
[0393] IR (KBr flak...
Embodiment 3
[0414] Example 3: N-{4-[3-(4-tert-butylbenzyl)ureidomethyl]-2-ethynyl-6-fluorophenyl}methanesulfonamide
[0415]
[0416] Step 1: tert-Butyl (4-amino-3-fluoro-5-trimethylsilylethynylbenzyl)carbamate
[0417] (4-Amino-3-fluoro-5-iodobenzyl)carbamate tert-butyl ester (100 mg, 0.273 mmol, 1 eq.), dichloro(di-triphenylphosphine), palladium (9.8 mg, 0.014 mmol, 0.05 eq.) and copper iodide (2.6 mg, 0.014 mmol, 0.05 eq.) in THF were placed in a 25 ml two necked round bottom flask and stirred at room temperature for 30 minutes. To this solution were added triethylamine (114.2 µl, 0.819 mmol, 3 eq.) and (trimethylsilyl)ethynyl (50.2 µl, 0.355 mmol, 1.3 eq.) and heated to reflux overnight. The reaction solution was concentrated under reduced pressure and subjected to column chromatography (n-hexane / ethyl acetate=5 / 1) to yield a brown liquid (84.0 mg, 91.4%).
[0418] IR (NaCl, pure, cm -1 ): 3459, 3360, 2965, 2148, 1698;
[0419] 1 H NMR (400MHz, CDCl 3 ): 7.00(d, 1H, J=0.8Hz),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com