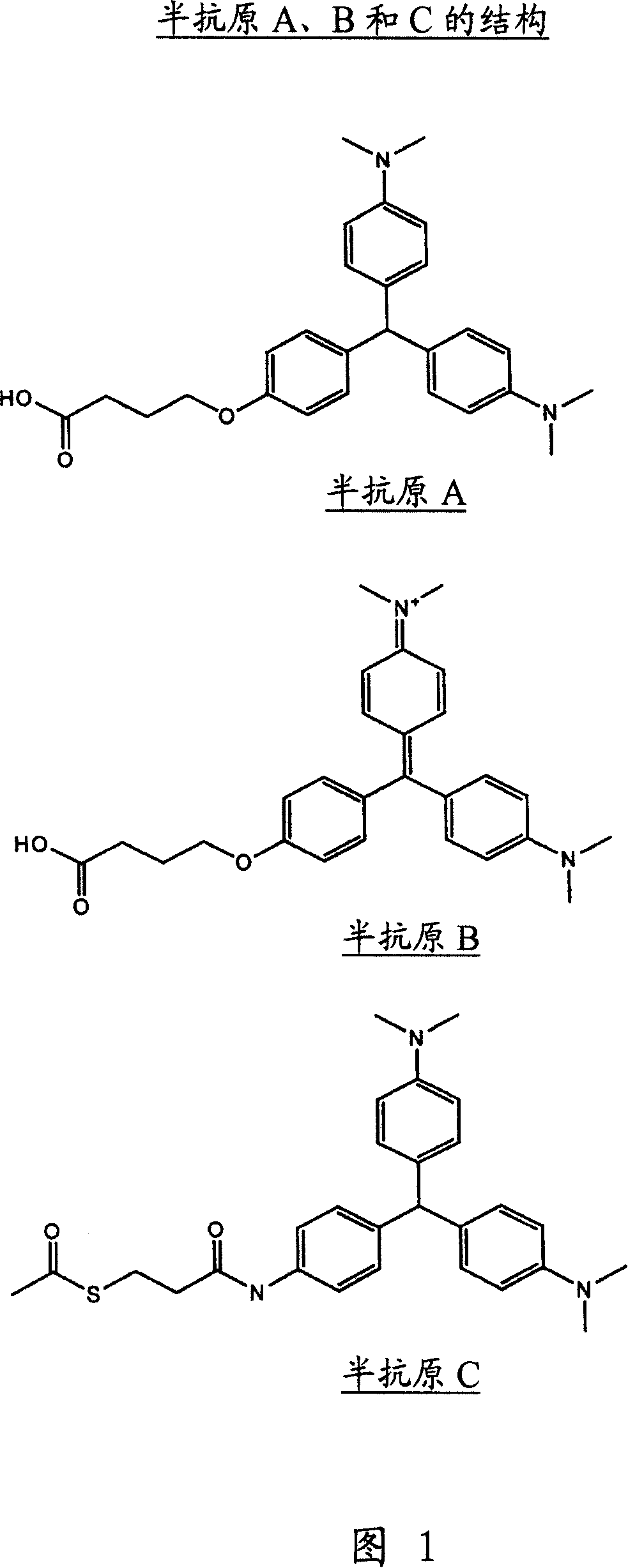
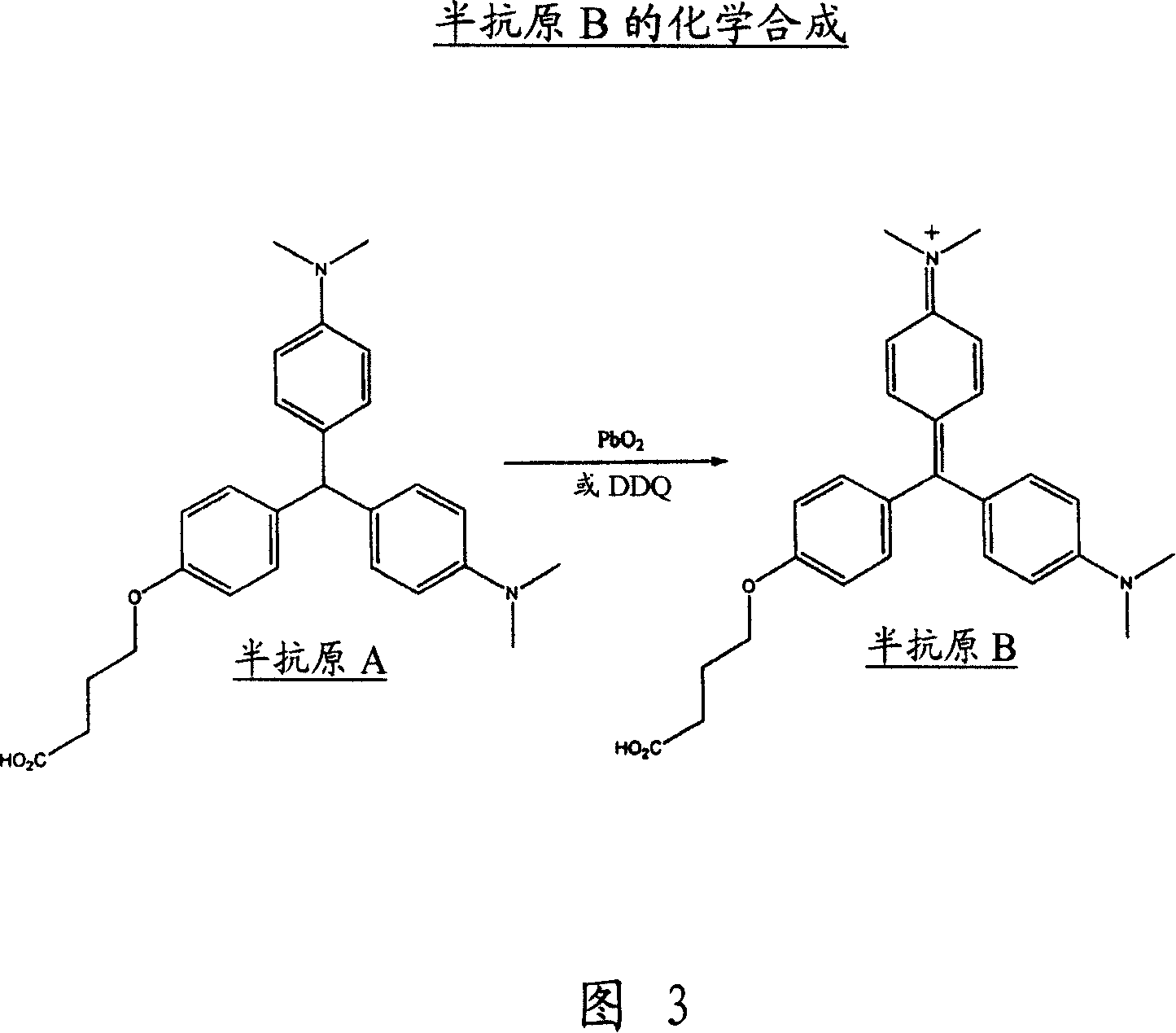
An immunoassay method and kit to leucomalachite green and malachite green
A technology for leuco malachite green and malachite green, which is applied in the field of immunoassay methods and kits for detecting or measuring leuco malachite green and malachite green, and can solve the problems of time-consuming experiment errors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
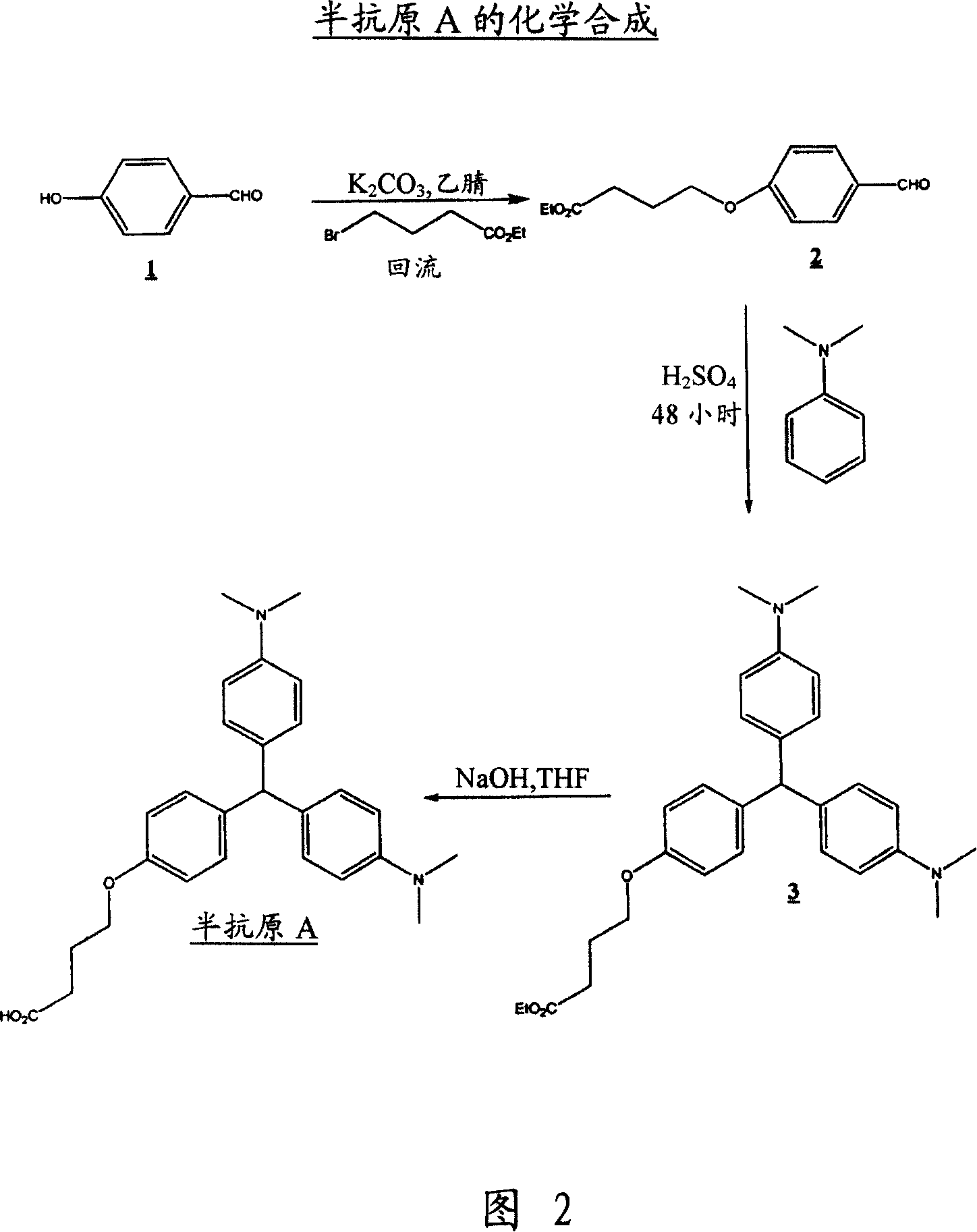
[0055] Embodiment 1: Preparation p-[3-(ethyl carboxyl) propoxy] benzaldehyde (2)
[0056] To a suspension of 4-hydroxybenzaldehyde, 1, (20 g, 164 mMol) and potassium carbonate (68 g, 492 mMol) in acetonitrile (300 ml) was added ethyl 4-bromobutyrate (35.2 ml, 246 mMol). The mixture was then heated to reflux overnight. After cooling to room temperature, the solid was filtered off and the solvent was removed in vacuo. Water (200ml) was then added to the residue and extracted with ethyl acetate (3x200ml). The combined organic phases were washed with brine (1x200ml), dried over sodium sulfate, filtered and evaporated to dryness. The resulting residue was purified by flash chromatography on silica gel using 30% ethyl acetate in hexane as eluent to give 2 (27 g, 69.8%) as a colorless oil.
[0057] IR spectrum (film): 1733.7, 1692.5, 1601.2, 1577.9, 1509.8, 1257.7, 1161.1cm -1 .
Embodiment 2
[0058] Embodiment 2: Preparation of [3-(ethyl carboxyl) propoxy] leuco malachite green (3)
[0059] To the mixture of p-[3-(ethylcarboxy)propoxy]benzaldehyde 2 (10g, 42.4mMol) and N,N-dimethylaniline (12.38g, 106mMol), concentrated sulfuric acid (2ml) was added, and the mixture Heat at 120°C for 48 hours. The solution was then cooled to room temperature, water (200ml) was added, the mixture was neutralized with solid sodium carbonate, extracted with ethyl acetate (3x100ml), washed with brine (1x100ml), dried over sodium sulfate, filtered and concentrated to dryness in vacuo. The resulting crude product was purified by flash chromatography on silica gel using 25% ethyl acetate in hexanes as eluent to give ester 3 (6.9 g, 35%) as an orange oil.
[0060] IR spectrum (film): 1735.3, 1612.2, 1518.3, 1242.6cm -1 .
Embodiment 3
[0061] Example 3: Preparation of p-(3-carboxypropoxy) leuco malachite green (hapten A)
[0062] To a solution of ester, 3, (8.4g, 18.75mMol) dissolved in a mixture of methanol and THF (2:1, 240ml) was added sodium hydroxide (2N, 40ml). The mixture was stirred at room temperature for 16 hours. The solvent was removed in vacuo and water (100ml) was added. The solution was neutralized to pH 7 by adding HCl (2N) and the resulting precipitate was collected by filtration and dried in a desiccator over phosphorus pentoxide. Hapten A was obtained as a blue / green solid (3.2 g, 40%).
[0063] NMR 13 C, solvent δ-MeOH (Figure 5): 178.8, 156.9, 148.9, 137.7, 133.6, 130.6, 129.9, 114.1, 112.9, 66.6, 54.2, 40.9, 30.9, 24.6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com